Zhang Q, Chen D, Lin J, Liao R, Tong W, Xu Z, Liu W
Characterization of NocL involved in thiopeptide nocathiacin I biosynthesis: a [4Fe-4S] cluster and the catalysis of a radical S-adenosylmethionine enzyme
▸ Abstract
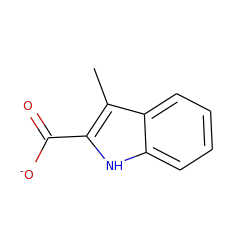
The radical S-adenosylmethionine (AdoMet) enzyme superfamily is remarkable at catalyzing chemically diverse and complex reactions. We have previously shown that NosL, which is involved in forming the indole side ring of the thiopeptide nosiheptide, is a radical AdoMet enzyme that processes L-Trp to afford 3-methyl-2-indolic acid (MIA) via an unusual fragmentation-recombination mechanism. We now report the expansion of the MIA synthase family by characterization of NocL, which is involved in nocathiacin I biosynthesis. EPR and UV-visible absorbance spectroscopic analyses demonstrated the interaction between L-Trp and the [4Fe-4S] cluster of NocL, leading to the assumption of nonspecific interaction of [4Fe-4S] cluster with other nucleophiles via the unique Fe site. This notion is supported by the finding of the heterogeneity in the [4Fe-4S] cluster of NocL in the absence of AdoMet, which was revealed by the EPR study at very low temperature. Furthermore, a free radical was observed by EPR during the catalysis, which is in good agreement with the hypothesis of a glycyl radical intermediate. Combined with the mutational analysis, these studies provide new insights into the function of the [4Fe-4S] cluster of radical AdoMet enzymes as well as the mechanism of the radical-mediated complex carbon chain rearrangement catalyzed by MIA synthase.
J Biol Chem
2011;286(24):21287-21294
| PubMed ID:
21454624
Zhang Q, Li Y, Chen D, Yu Y, Duan L, Shen B, Liu W
Radical-mediated enzymatic carbon chain fragmentation-recombination
▸ Abstract
The radical S-adenosylmethionine (S-AdoMet) superfamily contains thousands of proteins that catalyze highly diverse conversions, most of which are poorly understood, owing to a lack of information regarding chemical products and radical-dependent transformations. We here report that NosL, involved in forming the indole side ring of the thiopeptide nosiheptide (NOS), is a radical S-AdoMet 3-methyl-2-indolic acid (MIA) synthase. NosL catalyzed an unprecedented carbon chain reconstitution of L-tryptophan to give MIA, showing removal of the Cα-N unit and shift of the carboxylate to the indole ring. Dissection of the enzymatic process upon the identification of products and a putative glycyl intermediate uncovered a radical-mediated, unusual fragmentation-recombination reaction. This finding unveiled a key step in radical S-AdoMet enzyme-catalyzed structural rearrangements during complex biotransformations. Additionally, NosL tolerated fluorinated L-tryptophan as the substrate, allowing for production of a regiospecifically halogenated thiopeptide that has not been found among the more than 80 members of the naturally occurring thiopeptide family.
Nat Chem Biol
2011;7(3):154-160
| PubMed ID:
21240261
Nicolet Y, Zeppieri L, Amara P, Fontecilla-Camps JC
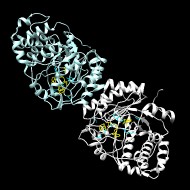
Crystal Structure of Tryptophan Lyase (NosL): Evidence for Radical Formation at the Amino Group of Tryptophan
▸ Abstract
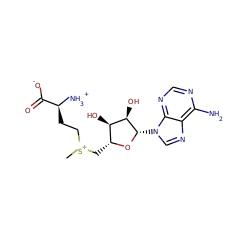
Streptomyces actuosus tryptophan lyase (NosL) is a radical SAM enzyme which catalyzes the synthesis of 3-methyl-2-indolic acid, a precursor in the synthesis of the promising antibiotic nosiheptide. The reaction involves cleavage of the tryptophan CαCβ bond and recombination of the amino-acid-derived -COOH fragment at the indole ring. Reported herein is the 1.8 Å resolution crystal structure of NosL complexed with its substrate. Unexpectedly, only one of the tryptophan amino hydrogen atoms is optimally placed for H abstraction by the SAM-derived 5'-deoxyadenosyl radical. This orientation, in turn, rules out the previously proposed delocalized indole radical as the species which undergoes CαCβ bond cleavage. Instead, stereochemical considerations indicate that the reactive intermediate is a (.) NH tryptophanyl radical. A structure-based amino acid sequence comparison of NosL with the tyrosine lyases ThiH and HydG strongly suggests that an equivalent (.) NH radical operates in the latter enzymes.
Angew Chem Int Ed Engl
2014;None(None):None-None
| PubMed ID:
25196319
Ji X, Li Y, Ding W, Zhang Q
Substrate-Tuned Catalysis of the Radical S-Adenosyl-L-Methionine Enzyme NosL Involved in Nosiheptide Biosynthesis
▸ Abstract
NosL is a radical S-adenosyl-L-methionine (SAM) enzyme that converts L-Trp to 3-methyl-2-indolic acid, a key intermediate in the biosynthesis of a thiopeptide antibiotic nosiheptide. In this work we investigated NosL catalysis by using a series of Trp analogues as the molecular probes. Using a benzofuran substrate 2-amino-3-(benzofuran-3-yl)propanoic acid (ABPA), we clearly demonstrated that the 5'-deoxyadenosyl (dAdo) radical-mediated hydrogen abstraction in NosL catalysis is not from the indole nitrogen but likely from the amino group of L-Trp. Unexpectedly, the major product of ABPA is a decarboxylated compound, indicating that NosL was transformed to a novel decarboxylase by an unnatural substrate. Furthermore, we showed that, for the first time to our knowledge, the dAdo radical-mediated hydrogen abstraction can occur from an alcohol hydroxy group. Our study demonstrates the intriguing promiscuity of NosL catalysis and highlights the potential of engineering radical SAM enzymes for novel activities.
Angew Chem Int Ed Engl
2015;None(None):None-None
| PubMed ID:
26138750
Bhandari DM, Xu H, Nicolet Y, Fontecilla-Camps JC, Begley TP
Tryptophan lyase (NosL): mechanistic insights from substrate analogs and mutagenesis
▸ Abstract
NosL is a member of a family of radical S-adenosylmethionine (SAM) enzymes that catalyze the cleavage of the Cα-Cβ bond of aromatic amino acids. In this paper, we describe a set of experiments with substrate analogs and mutants to probe the early steps of the NosL mechanism. We provide biochemical evidence in support of the structural studies showing that the 5'-deoxyadenosyl radical abstracts a hydrogen atom from the amino group of tryptophan. We demonstrate that D-tryptophan is a substrate for NosL but shows relaxed regio control of the first β-scission reaction. Mutagenesis studies confirm that Arg323 is important for controlling the regiochemistry of the β-scission reaction and that Ser340 binds the substrate by hydrogen bonding to the indolic N1.
Biochemistry
2015;None(None):None-None
| PubMed ID:
26204056
Sicoli G, Mouesca JM, Zeppieri L, Amara P, Martin L, Barra AL, Fontecilla-Camps JC, Gambarelli S, Nicolet Y
Fine-tuning of a radical-based reaction by radical S-adenosyl-L-methionine tryptophan lyase
▸ Abstract
The radical S-adenosyl-L-methionine tryptophan lyase NosL converts L-tryptophan into 3-methylindolic acid, which is a precursor in the synthesis of the thiopeptide antibiotic nosiheptide. Using electron paramagnetic resonance spectroscopy and multiple L-tryptophan isotopologues, we trapped and characterized radical intermediates that indicate a carboxyl fragment migration mechanism for NosL. This is in contrast to a proposed fragmentation-recombination mechanism that implied Cα-Cβ bond cleavage of L-tryptophan. Although NosL resembles related tyrosine lyases, subtle substrate motions in its active site are responsible for a fine-tuned radical chemistry, which selects the Cα-C bond for disruption. This mechanism highlights evolutionary adaptation to structural constraints in proteins as a route to alternative enzyme function.
Science
2016;351(6279):1320-1323
| PubMed ID:
26989252
Ji X, Li Y, Jia Y, Ding W, Zhang Q
Mechanistic Insights into the Radical S-adenosyl-l-methionine Enzyme NosL From a Substrate Analogue and the Shunt Products
▸ Abstract
The radical S-adenosyl-l-methionine (SAM) enzyme NosL catalyzes the transformation of l-tryptophan into 3-methyl-2-indolic acid (MIA), which is a key intermediate in the biosynthesis of a clinically interesting antibiotic nosiheptide. NosL catalysis was investigated by using the substrate analogue 2-methyl-3-(indol-3-yl)propanoic acid (MIPA), which can be converted into MIA by NosL. Biochemical assays with different MIPA isotopomers in D2 O and H2 O unambiguously indicated that the 5'-deoxyadenosyl (dAdo)-radical-mediated hydrogen abstraction is from the amino group of l-tryptophan and not a protein residue. Surprisingly, the dAdo-radical-mediated hydrogen abstraction occurs at two different sites of MIPA, thereby partitioning the substrate into different reaction pathways. Together with identification of an α,β-unsaturated ketone shunt product, our study provides valuable mechanistic insight into NosL catalysis and highlights the remarkable catalytic flexibility of radical SAM enzymes.
Angew Chem Int Ed Engl
2016;55(10):3334-3337
| PubMed ID:
26837062
Ding W, Ji X, Li Y, Zhang Q
Catalytic Promiscuity of the Radical S-adenosyl-L-methionine Enzyme NosL
▸ Abstract
Catalytic promiscuity plays a key role in enzyme evolution and the acquisition of novel biological functions. Because of the high reactivity of radical species, in our view enzymes involving radical-mediated mechanisms could intrinsically be more prone to catalytic promiscuity. This mini-review summarizes the recent advances in the study of NosL, a radical S-adenosyl-L-methionine (SAM)-dependent L-tryptophan (L-Trp) lyase. We demonstrate here the interesting chemistry and remarkable catalytic promiscuity of NosL, and attempt to highlight the high evolvability of radical SAM enzymes and the potential to engineer these enzymes for novel and improved activities.
Front Chem
2016;4(None):2-27
| PubMed ID:
27446906
Ji X, Liu WQ, Yuan S, Yin Y, Ding W, Zhang Q
Mechanistic study of the radical SAM-dependent amine dehydrogenation reactions
▸ Abstract
The radical SAM enzyme NosL catalyzes the conversion of l-Trp to 3-methyl-2-indolic acid, and this reaction is initiated by the 5'-deoxyadenosyl (dAdo) radical-mediated hydrogen abstraction from the l-Trp amino group. We demonstrate here that when d-Trp was used in the NosL reaction, hydrogen abstraction occurs promiscuously at both the amino group and Cα of d-Trp. These results inspired us to establish the detailed mechanism of l-Trp amine dehydrogenation catalyzed by a NosL mutant, and to engineer a novel radical SAM-dependent l-Tyr amine dehydrogenase from the thiamine biosynthesis enzyme ThiH.
Chem Commun (Camb)
2016;52(69):10555-10558
| PubMed ID:
27492649