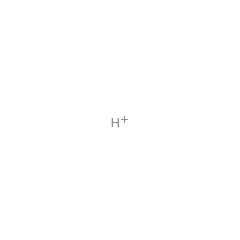
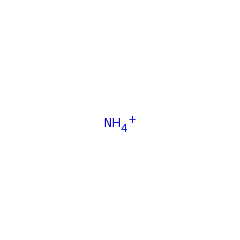
The biosynthesis of the deazapurine base preQ(0), is catalysed by the successive action of four enzymes (GCH I, QueD, QueE and QueC). The pathway includes the conversion of the biosynthetic intermediate, 6-carboxy-5,6,7,8-tetrahydropterin, to a novel intermediate, 7-carboxy-7-deazaguanine (CDG), by an unusual transformation catalyzed by Bacillus subtilis QueE, a member of the radical SAM enzyme superfamily. The mechanism proposed by McCarty et al. showing that the SAM is utilised catalytic and that the reaction is Mg(II) dependent.
McCarty RM, Somogyi A, Lin G, Jacobsen NE, Bandarian V
The deazapurine biosynthetic pathway revealed: in vitro enzymatic synthesis of PreQ(0) from guanosine 5'-triphosphate in four steps
▸ Abstract
Deazapurine-containing secondary metabolites comprise a broad range of structurally diverse nucleoside analogues found throughout biology, including various antibiotics produced by species of Streptomyces bacteria and the hypermodified tRNA bases queuosine and archaeosine. Despite early interest in deazapurines as antibiotic, antiviral, and antineoplastic agents, the biosynthetic route toward deazapurine production has remained largely elusive for more than 40 years. Here we present the first in vitro preparation of the deazapurine base preQ(0), by the successive action of four enzymes. The pathway includes the conversion of the recently identified biosynthetic intermediate, 6-carboxy-5,6,7,8-tetrahydropterin, to a novel intermediate, 7-carboxy-7-deazaguanine (CDG), by an unusual transformation catalyzed by Bacillus subtilis QueE, a member of the radical SAM enzyme superfamily. The carboxylate moiety on CDG is converted subsequently to a nitrile to yield preQ(0) by either B. subtilis QueC or Streptomyces rimosus ToyM in an ATP-dependent reaction, in which ammonia serves as the nitrogen source. The results presented here are consistent with early radiotracer studies on deazapurine biosynthesis and provide a unified pathway for the production of deazapurines in nature.
Biochemistry
2009;48(18):3847-3852
| PubMed ID:
19354300
McCarty RM, Krebs C, Bandarian V
Spectroscopic, steady-state kinetic, and mechanistic characterization of the radical SAM enzyme QueE, which catalyzes a complex cyclization reaction in the biosynthesis of 7-deazapurines
▸ Abstract
7-Carboxy-7-deazaguanine (CDG) synthase (QueE) catalyzes the complex heterocyclic radical-mediated conversion of 6-carboxy-5,6,7,8-tetrahydropterin (CPH(4)) to CDG in the third step of the biosynthetic pathway to all 7-deazapurines. Here we present a detailed characterization of QueE from Bacillus subtilis to delineate the mechanism of conversion of CPH(4) to CDG. QueE is a member of the radical S-adenosyl-l-methionine (SAM) superfamily, all of which use a bound [4Fe-4S](+) cluster to catalyze the reductive cleavage of the SAM cofactor to generate methionine and a 5'-deoxyadenosyl radical (5'-dAdo.), which initiates enzymatic transformations requiring hydrogen atom abstraction. The ultraviolet-visible, electron paramagnetic resonance, and Mössbauer spectroscopic features of the homodimeric QueE point to the presence of a single [4Fe-4S] cluster per monomer. Steady-state kinetic experiments indicate a K(m) of 20 ± 7 μM for CPH(4) and a k(cat) of 5.4 +- 1.2 min(-1) for the overall transformation. The kinetically determined K(app) for SAM is 45 +- 1 μM. QueE is also magnesium-dependent and exhibits a K(app) for the divalent metal ion of 0.21 ± 0.03 mM. The SAM cofactor supports multiple turnovers, indicating that it is regenerated at the end of each catalytic cycle. The mechanism of rearrangement of QueE was probed with CPH(4) isotopologs containing deuterium at C-6 or the two prochiral positions at C-7. These studies implicate 5'-dAdo. as the initiator of the ring contraction reaction catalyzed by QueE by abstraction of the H atom from C-6 of CPH(4).
Biochemistry
2013;52(1):188-198
| PubMed ID:
23194065
Dowling DP, Bruender NA, Young AP, McCarty RM, Bandarian V, Drennan CL
Radical SAM enzyme QueE defines a new minimal core fold and metal-dependent mechanism
▸ Abstract
7-carboxy-7-deazaguanine synthase (QueE) catalyzes a key S-adenosyl-L-methionine (AdoMet)- and Mg(2+)-dependent radical-mediated ring contraction step, which is common to the biosynthetic pathways of all deazapurine-containing compounds. QueE is a member of the AdoMet radical superfamily, which employs the 5'-deoxyadenosyl radical from reductive cleavage of AdoMet to initiate chemistry. To provide a mechanistic rationale for this elaborate transformation, we present the crystal structure of a QueE along with structures of pre- and post-turnover states. We find that substrate binds perpendicular to the [4Fe-4S]-bound AdoMet, exposing its C6 hydrogen atom for abstraction and generating the binding site for Mg(2+), which coordinates directly to the substrate. The Burkholderia multivorans structure reported here varies from all other previously characterized members of the AdoMet radical superfamily in that it contains a hypermodified (β6/α3) protein core and an expanded cluster-binding motif, CX14CX2C.
Nat Chem Biol
2014;10(2):106-112
| PubMed ID:
24362703
Reader JS, Metzgar D, Schimmel P, de Crécy-Lagard V
Identification of four genes necessary for biosynthesis of the modified nucleoside queuosine
▸ Abstract
Queuosine (Q) is a hypermodified 7-deazaguanosine nucleoside located in the anticodon wobble position of four amino acid-specific tRNAs. In bacteria, Q is produced de novo from GTP via the 7-deazaguanosine precursor preQ1 (7-aminoethyl 7-deazaguanine) by an uncharacterized pathway. PreQ1 is subsequently transferred to its specific tRNA by a tRNA-guanine transglycosylase (TGT) and then further modified in situ to produce Q. Here we use comparative genomics to implicate four gene families (best exemplified by the B. subtilis operon ykvJKLM) as candidates in the preQ1 biosynthetic pathway. Deletions were constructed in genes for each of the four orthologs in Acinetobacter. High pressure liquid chromatography analysis showed the Q nucleoside was absent from the tRNAs of each of four deletion strains. Electrospray ionization mass spectrometry confirmed the absence of Q in each mutant strain. Finally, introduction of the Bacillus subtilis ykvJKLM operon in trans complemented the Q deficiency of the two deletion mutants that were tested. Thus, the products of these four genes (named queC, -D, -E, and -F) are essential for the Q biosynthetic pathway.
J Biol Chem
2004;279(8):6280-6285
| PubMed ID:
14660578
Bruender NA, Young AP, Bandarian V
Chemical and Biological Reduction of the Radical SAM Enzyme CPH4 Synthase
▸ Abstract
The radical S-adenosyl-L-methionine (SAM) superfamily is a large and growing group of enzymes that conduct complex radical-mediated transformations. A one-electron reduction of SAM via the +1 state of the cubane [4Fe-4S] cluster generates a 5'-deoxyadenosyl radical, which initiates turnover. The [4Fe-4S] cluster must be reduced from its resting +2 state to the catalytically active +1 oxidation state by an electron. In practice, dithionite or the Escherichia coli flavodoxin (EcFldA)/ferredoxin (flavodoxin):NADP(+) oxidoreductase (Fpr)/NADPH system is used. Herein, we present a systematic investigation of the reductive activation of the radical SAM enzyme CDG synthase (BsQueE) from Bacillus subtilis comparing biological and chemical reductants. These data show that either of the flavodoxin homologues encoded by the B. subtilis genome, BsYkuN or BsYkuP, as well as a series of small molecule redox mediators, supports BsQueE activity. With dithionite as a reductant, the activity of BsQueE is ~75-fold greater in the presence of BsYkuN and BsYkuP compared to that in the presence of dithionite alone. By contrast, EcFldA supports turnover to ~10-fold greater levels than dithionite alone under the same conditions. Comparing the ratio of the rate of turnover to the apparent binding constant for the flavodoxin homologues reveals 10- and 240-fold preferences for BsYkuN over BsYkuP and EcFldA, respectively. The differential activation of the enzyme cannot be explained by the abortive cleavage of SAM. We conclude from these observations that the differential activation of BsQueE by Fld homologues may reside in the details of the interaction between the flavodoxin and the radical SAM enzyme.
Biochemistry
2015;54(18):2903-2910
| PubMed ID:
25933252