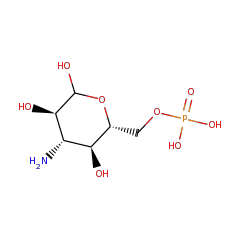
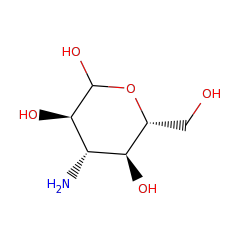
Top Level Name
⌊ Superfamily (core) Haloacid Dehalogenase
⌊ Subgroup C2.B: Phosphomannomutase and Phosphatase Like
⌊ C2.B.1: Sucrose Phosphatase Like
⌊ FunctionalDomain C2.B.1: Sucrose Phosphatase Like (ID 105187)
No Notes.
| Superfamily Assignment Evidence Code(s) | ISS |
| This entry was last updated on | Nov. 22, 2017 |
References to Other Databases
Genbank
| Species | GI | Accession | Proteome |
|---|---|---|---|
| Bacillus subtilis subsp. subtilis str. 168 Taxon ID: 224308 | 237640626 | PRP URP | |
| Bacillus subtilis subsp. subtilis str. 168 Taxon ID: 224308 | 237640625 | PRP URP | |
| Bacillus subtilis subsp. subtilis str. 168 Taxon ID: 224308 | 237640624 | PRP URP | |
| Bacillus subtilis subsp. subtilis str. 168 Taxon ID: 224308 | 237640623 | PRP URP | |
| Show All | |||
Uniprot
| Protein Name | Accession | EC Number
 |
Identifier |
|---|---|---|---|
| Kanosamine-6-phosphate phosphatase | O07565 | 3.1.3.92 | NTDB_BACSU (Swiss-Prot) |
Length of Enzyme (full-length): 289 | Length of Functional Domain: 263
SNAXLLSKKSEYKTLSTVEHPQYIVFCDFDETYFPHTIDEQKQQDIYELEDYLEQKSKDG
ELIIGWVTGSSIESILDKXGRGKFRYFPHFIASDLGTEITYFSEHNFGQQDNKWNSRINE
GFSKEKVEKLVKQLHENHNILLNPQTQLGKSRYKHNFYYQEQDEINDKKNLLAIEKICEE
YGVSVNINRCNPLAGDPEDSYDVDFIPIGTGKNEIVTFXLEKYNLNTERAIAFGDSGNDV
RXLQTVGNGYLLKNATQEAKNLHNLITDSEYSKGITNTLKKLIGFXRRK
Conserved catalytic residues (as determined by automated alignment to family, subgroup, or superfamily HMMs) are shown with teal highlighting . Conserved catalytic residues which do not matched the Conserved Alignment Residue are shown with maroon highlighting . Information regarding their function can be found in the Conserved Residues section below.