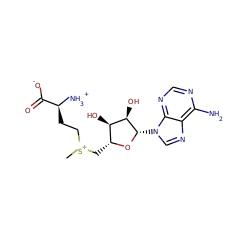
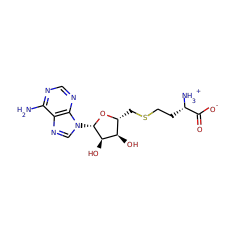
Responsible for the methylation of gentamicin at the C6 position. It has recently been shown that GenK acts at the X2 Branch Point and is essential for the production of G418 and Gentamicins C2a, C2, and C1. It also acts (with the same function) at the A and A2 branch points.
Kim JY, Suh JW, Kang SH, Phan TH, Park SH, Kwon HJ
Gene inactivation study of gntE reveals its role in the first step of pseudotrisaccharide modifications in gentamicin biosynthesis
▸ Abstract
A gene inactivation study was performed on gntE, a member of the gentamicin biosynthetic gene cluster in Micromonospora echinospora. Computer-aided homology analysis predicts a methyltransferase-related cobalamin-binding domain and a radical S-adenosylmethionine domain in GntE. It is also found that there is no gntE homolog within other aminoglycoside biosynthetic gene clusters. Inactivation of gntE was achieved in both M. echinospora ATCC 15835 and a gentamicin high-producer GMC106. High-performance liquid chromatographic analysis, coupled with mass spectrometry, revealed that gntE mutants accumulated gentamicin A2 and its derivative with a methyl group installed on the glucoamine moiety. This result substantiated that GntE participates in the first step of pseudotrisaccharide modifications in gentamicin biosynthesis, though the catalytic nature of this unusual oxidoreductase/methyltransferase candidate is not resolved. The present gene inactivation study also demonstrates that targeted genetic engineering can be applied to produce specific gentamicin structures and potentially new gentamicin derivatives in M. echinospora.
Biochem Biophys Res Commun
2008;372(4):730-734
| PubMed ID:
18533111
Kim HJ, McCarty RM, Ogasawara Y, Liu YN, Mansoorabadi SO, Levieux J, Liu HW
GenK-Catalyzed C-6' Methylation in the Biosynthesis of Gentamicin: Isolation and Characterization of a Cobalamin-Dependent Radical SAM Enzyme
▸ Abstract
The existence of cobalamin (Cbl)-dependent enzymes that are members of the radical S-adenosyl-l-methionine (SAM) superfamily was previously predicted on the basis of bioinformatic analysis. A number of these are Cbl-dependent methyltransferases, but the details surrounding their reaction mechanisms have remained unclear. In this report we demonstrate the in vitro activity of GenK, a Cbl-dependent radical SAM enzyme that methylates an unactivated sp3 carbon during the biosynthesis of gentamicin, an aminoglycoside antibiotic. Experiments to investigate the stoichiometry of the GenK reaction revealed that 1 equiv each of 5'-deoxyadenosine and S-adenosyl-homocysteine are produced for each methylation reaction catalyzed by GenK. Furthermore, isotope-labeling experiments demonstrate that the S-methyl group from SAM is transferred to Cbl and the aminoglycoside product during the course of the reaction. On the basis of these results, one mechanistic possibility for the GenK reaction can be ruled out, and further questions regarding the mechanisms of Cbl-dependent radical SAM methyltransferases, in general, are discussed.
J Am Chem Soc
2013;None(None):None-None
| PubMed ID:
23679096
Guo J, Huang F, Huang C, Duan X, Jian X, Leeper F, Deng Z, Leadlay PF, Sun Y
Specificity and Promiscuity at the Branch Point in Gentamicin Biosynthesis
▸ Abstract
Gentamicin C complex is a mixture of aminoglycoside antibiotics used to treat severe Gram-negative bacterial infections. We report here key features of the late-stage biosynthesis of gentamicins. We show that the intermediate gentamicin X2, a known substrate for C-methylation at C-6' to form G418 catalyzed by the radical SAM-dependent enzyme GenK, may instead undergo oxidation at C-6' to form an aldehyde, catalyzed by the flavin-linked dehydrogenase GenQ. Surprisingly, GenQ acts in both branches of the pathway, likewise oxidizing G418 to an analogous ketone. Amination of these intermediates, catalyzed mainly by aminotransferase GenB1, produces the known intermediates JI-20A and JI-20B, respectively. Other pyridoxal phosphate-dependent enzymes (GenB3 and GenB4) act in enigmatic dehydroxylation steps that convert JI-20A and JI-20B into the gentamicin C complex or (GenB2) catalyze the epimerization of gentamicin C2a into gentamicin C2.
Chem Biol
2014;None(None):None-None
| PubMed ID:
24746560
Huang C, Huang F, Moison E, Guo J, Jian X, Duan X, Deng Z, Leadlay PF, Sun Y
Delineating the biosynthesis of gentamicin x2, the common precursor of the gentamicin C antibiotic complex
▸ Abstract
Gentamicin C complex is a mixture of aminoglycoside antibiotics used worldwide to treat severe Gram-negative bacterial infections. Despite its clinical importance, the enzymology of its biosynthetic pathway has remained obscure. We report here insights into the four enzyme-catalyzed steps that lead from the first-formed pseudotrisaccharide gentamicin A2 to gentamicin X2, the last common intermediate for all components of the C complex. We have used both targeted mutations of individual genes and reconstitution of portions of the pathway in vitro to show that the secondary alcohol function at C-3″ of A2 is first converted to an amine, catalyzed by the tandem operation of oxidoreductase GenD2 and transaminase GenS2. The amine is then specifically methylated by the S-adenosyl-l-methionine (SAM)-dependent N-methyltransferase GenN to form gentamicin A. Finally, C-methylation at C-4″ to form gentamicin X2 is catalyzed by the radical SAM-dependent and cobalamin-dependent enzyme GenD1.
Chem Biol
2015;22(2):251-261
| PubMed ID:
25641167