Top Level Name
⌊ Superfamily (core) Radical SAM
⌊ Subgroup B12-binding domain containing
⌊ Family P-methyltransferase (PhpK-like)
| Total |
100%  |
<100%  |
|||
| Functional domains | 12 | 11 | 1 | ||
| UniProtKB | 14 | 13 | 1 | ||
| GI | 35 | 32 | 3 | ||
| Structures | 0 | ||||
| Reactions | 1 | ||||
| Functional domains of this family were last updated on June 10, 2017 | |||||
| New functional domains were last added to this family on June 22, 2014 | |||||
Vitamin B12 dependent methylation of a phosphinate phosphorus atom. Enzymes in this family utilise exogenous methylcob(III)alamin to transfer a methyl group to 2-acetylamino-4-hydroxyphosphinylbutanoate (N-acetylde-methylphosphinothricin) to form 2-acetylamino-4-hydroxymethylphosphinylbutanoate (N-acetylphosphinothricin). To date, this is the only carbon-phosphorus−carbon linkage known to occur in Nature.
Werner WJ, Allen KD, Hu K, Helms GL, Chen BS, Wang SC
In vitro phosphinate methylation by PhpK from Kitasatospora phosalacinea
▸ Abstract
Biochemistry 2011;50(42):8986-8988 | PubMed ID: 21950770
See also review article http://onlinelibrary.wiley.com/doi/10.1002/cbic.201200762/pdf
Static File Downloads
| File Name | Description | Parameters | Stats |
|---|---|---|---|
| network.fam328.bs60.mek250K.xgmml | One node per sequence network | min bit score = 60 max edge count = 250K |
size = 52K num_edges = 66 num_nodes = 12 |
| sfld_alignment_fam328.msa | Annotated Sequence Alignment, Stockholm format | 8 sequences size: 6.7K |
Catalyzed Reaction(s)
| 2 | 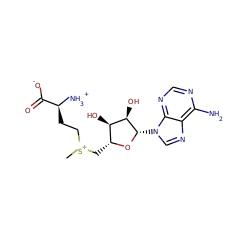 |
+ | 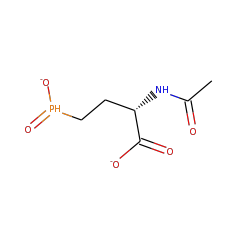 |
 |
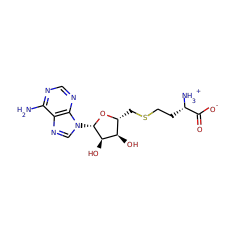 |
+ |  |
+ |  |
+ |  |
|
| S-adenosyl-L-methionine zwitterion 59789 |
N-Acetyldemethylphosphinothricinate (2-) 86401 |
S-adenosyl-L-homocysteine zwitterion 57856 |
L-methionine zwitterion 57844 |
5'-deoxyadenosine 17319 |
N-acetylphosphinatothricinate(2-) 58879 |
EC: 2.1.1.- | IntEnz: 2.1.1.- | Kegg: 2.1.1.- | BioCyc: 2.1.1.- | BRENDA: 2.1.1.- |


