Top Level Name
⌊ Superfamily (core) Radical SAM
⌊ Subgroup SPASM/twitch domain containing
⌊ main SPASM domain-containing
⌊ thioether bond formation requiring one auxiliary iron-sulfur cluster
⌊ Family antilisterial bacteriocin subtilosin biosynthesis protein (AlbA-like)
| Total |
100%  |
<100%  |
|||
| Functional domains | 49 | 49 | 0 | ||
| UniProtKB | 87 | 87 | 0 | ||
| GI | 187 | 187 | 0 | ||
| Structures | 0 | ||||
| Reactions | 2 | ||||
| Functional domains of this family were last updated on June 10, 2017 | |||||
| New functional domains were last added to this family on June 22, 2014 | |||||
This radical S-adenosylmethionine enzyme is encoded by the sbo-alb operon and comprises two [4Fe-4S] clusters. One [4Fe-4S] cluster is coordinated by the prototypical C[X]3C[X]2C motif and is responsible for the observed S-adenosylmethionine cleavage reaction, whereas the second [4Fe-4S] cluster is required for the generation of all three thioether linkages in subtilosin A. The mature subtilosin has been shown to display spermicidal activity and antimicrobial activity against a range og Gram-positive and Gram-negative bacteria.
Flühe L, Knappe TA, Gattner MJ, Schäfer A, Burghaus O, Linne U, Marahiel MA
The radical SAM enzyme AlbA catalyzes thioether bond formation in subtilosin A
▸ Abstract
Nat Chem Biol 2012;8(4):350-357 | PubMed ID: 22366720
Benjdia A, Guillot A, Lefranc B, Vaudry H, Leprince J, Berteau O
Thioether bond formation by SPASM domain radical SAM enzymes: Cα H-atom abstraction in subtilosin A biosynthesis
▸ Abstract
Chem Commun (Camb) 2016;52(37):6249-6252 | PubMed ID: 27087315
Initially, an electron from an external reducing agent (for example, sodium dithionite) is transferred to the first [4Fe-4S] cluster (coordinated by the CXXXCXXC motif) to convert it into its active reduced form. Subsequently, coordinated SAM is reductively cleaved into methionine and the 5'-dA radical by the transfer of the electron onto SAM. SboA coordinates with the unique iron of the second [4Fe-4S] cluster (coordinated by Cys408, Cys414, Cys417) via a thiol group of a cysteine residue, which is deprotonated upon binding to the cluster. The generated 5'-dA radical abstracts a hydrogen atom from the a-carbon of the threonine or phenylalanine residues. In the last step, the carbon-centered radical attacks the coordinated sulfur atom by forming the thioether bond, and the Fe-S cluster acts as the acceptor of the second electron. This reaction step is similar to the key aspect of the BioB- and isopenicillin N synthase–catalyzed reactions, which involve the attack of a carbon-centered radical on a sulfur atom that is ligated to an iron atom and acts as an electron acceptor. The reduced form of the second cluster may be able to transfer the electron to the first cluster via intramolecular electron channeling, converting both clusters into their active forms and preparing the enzyme for a second turnover. Alternatively, the electron from the second cluster may be transferred to an external one electron acceptor, in which case the first cluster must be reactivated by an external electron source for a second turnover, which is observed in the peptide modification assays when AlbA is used in catalytic amounts.
Static File Downloads
| File Name | Description | Parameters | Stats |
|---|---|---|---|
| network.fam315.bs60.mek250K.xgmml | One node per sequence network | min bit score = 60 max edge count = 250K |
size = 693K num_edges = 1176 num_nodes = 49 |
| sfld_alignment_fam315.msa | Annotated Sequence Alignment, Stockholm format | 20 sequences size: 14K |
Catalyzed Reaction(s)
thioether cross-link between Cys and Thr
 |
+ |  |
+ |  |
 |
 |
+ |  |
+ |  |
||
| cysteine residue 32460 |
L-threonine residue 30013 |
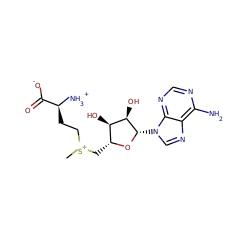
S-adenosyl-L-methionine zwitterion 59789 |
Cys-Thr thioether 132254 |
5'-deoxyadenosine 17319 |
L-methionine zwitterion 57844 |
EC: | IntEnz: | Kegg: | BioCyc: | BRENDA: |
thioether cross-link between Cys and Phe
 |
+ |  |
+ |  |
 |
 |
+ |  |
+ |  |
||
| cysteine residue 32460 |
phenylalanine residue 32503 |
S-adenosyl-L-methionine zwitterion 59789 |
Cys-Phe thioether 132409 |
5'-deoxyadenosine 17319 |
L-methionine zwitterion 57844 |
EC: | IntEnz: | Kegg: | BioCyc: | BRENDA: |


