Zhang Q, van der Donk WA, Liu W
Radical-mediated enzymatic methylation: a tale of two SAMS
▸ Abstract
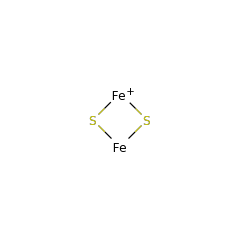
Methylation is an essential and ubiquitous reaction that plays an important role in a wide range of biological processes. Most biological methylations use S-adenosylmethionine (SAM) as the methyl donor and proceed via an S(N)2 displacement mechanism. However, researchers have discovered an increasing number of methylations that involve radical chemistry. The enzymes known to catalyze these reactions all belong to the radical SAM superfamily. This family of enzymes utilizes a specialized [4Fe-4S] cluster for reductive cleavage of SAM to yield a highly reactive 5'-deoxyadenosyl (dAdo) radical. Radical chemistry is then imposed on a variety of organic substrates, leading to a diverse array of transformations. Until recently, researchers had not fully understood how these enzymes employ radical chemistry to mediate a methyl transfer reaction. Sequence analyses reveal that the currently identified radical SAM methyltransferases (RSMTs) can be grouped into three classes, which appear distinct in protein architecture and mechanism. Class A RSMTs mainly include the rRNA methyltransferases RlmN and Cfr from various origins. As exemplified by Escherichia coli RlmN, these proteins have a single canonical radical SAM core domain that includes an (βα)(6) partial barrel most similar to that of pyruvate formate lyase-activase. The exciting recent studies on RlmN and Cfr are beginning to provide insights into the intriguing chemistry of class A RSMTs. These enzymes utilize a methylene radical generated on a unique methylated cysteine residue. However, based on the variety of substrates used by the other classes of RSMTs, alternative mechanisms are likely to be discovered. Class B RSMTs contain a proposed N-terminal cobalamin binding domain in addition to a radical SAM domain at the C-terminus. This class of proteins methylates diverse substrates at inert sp(3) carbons, aromatic heterocycles, and phosphinates, possibly involving a cobalamin-mediated methyl transfer process. Class C RSMTs share significant sequence similarity with coproporphyrinogen III oxidase HemN. Despite methylating similar substrates (aromatic heterocycles), class C RSMTs likely employ a mechanism distinct from that of class A because two conserved cysteines that are required for class A are typically not found in class C RSMTs. Class A and class B enzymes probably share the use of two molecules of SAM: one to generate a dAdo radical and one to provide the methyl group to the substrate. In class A, a cysteine would act as a conduit of the methyl group whereas in class B cobalamin may serve this purpose. Currently no clues are available regarding the mechanism of class C RSMTs, but the sequence similarities between its members and HemN and the observation that HemN binds two SAM molecules suggest that class C enzymes could use two SAM molecules for catalysis. The diverse strategies for using two SAM molecules reflect the rich chemistry of radical-mediated methylation reactions and the remarkable versatility of the radical SAM superfamily.
Acc Chem Res
2012;45(4):555-564
| PubMed ID:
22097883
Yan F, Fujimori DG
RNA methylation by radical SAM enzymes RlmN and Cfr proceeds via methylene transfer and hydride shift
▸ Abstract
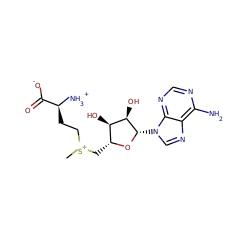
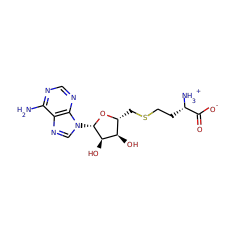
RlmN and Cfr are Radical SAM enzymes that modify a single adenosine nucleotide--A2503--in 23S ribosomal RNA. This nucleotide is positioned within the peptidyl transferase center of the ribosome, which is a target of numerous antibiotics. An unusual feature of these enzymes is their ability to carry out methylation of amidine carbons of the adenosine substrate. To gain insight into the mechanism of methylation catalyzed by RlmN and Cfr, deuterium labeling experiments were carried out. These experiments demonstrate that the newly introduced methyl group is assembled from an S-adenosyl-L-methionine (SAM)-derived methylene fragment and a hydrogen atom that had migrated from the substrate amidine carbon. Rather than activating the adenosine nucleotide of the substrate by hydrogen atom abstraction from an amidine carbon, the 5'-deoxyadenosyl radical abstracts hydrogen from the second equivalent of SAM to form the SAM-derived radical cation. This species, or its corresponding sulfur ylide, subsequently adds into the substrate, initiating hydride shift and S-adenosylhomocysteine elimination to complete the formation of the methyl group. These findings indicate that rather than acting as methyltransferases, RlmN and Cfr are methyl synthases. Together with the previously described 5'-deoxyadenosyl and 3-amino-3-carboxypropyl radicals, these findings demonstrate that all three carbon atoms attached to the sulfonium center in SAM can serve as precursors to carbon-derived radicals in enzymatic reactions.
Proc Natl Acad Sci U S A
2011;108(10):3930-3934
| PubMed ID:
21368151
Yan F, LaMarre JM, Röhrich R, Wiesner J, Jomaa H, Mankin AS, Fujimori DG
RlmN and Cfr are radical SAM enzymes involved in methylation of ribosomal RNA
▸ Abstract
Posttranscriptional modifications of ribosomal RNA (rRNA) nucleotides are a common mechanism of modulating the ribosome's function and conferring bacterial resistance to ribosome-targeting antibiotics. One such modification is methylation of an adenosine nucleotide within the peptidyl transferase center of the ribosome mediated by the endogenous methyltransferase RlmN and its evolutionarily related resistance enzyme Cfr. These methyltransferases catalyze methyl transfer to aromatic carbon atoms of the adenosine within a complex 23S rRNA substrate to form the 2,8-dimethylated product. RlmN and Cfr are members of the Radical SAM superfamily and contain the characteristic cysteine-rich CX(3)CX(2)C motif. We demonstrate that both enzymes are capable of accommodating the requisite [4Fe-4S] cluster. S-Adenosylmethionine (SAM) is both the methyl donor and the source of a 5'-deoxyadenosyl radical, which activates the substrate for methylation. Detailed analyses of the rRNA requirements show that the enzymes can utilize protein-free 23S rRNA as a substrate, but not the fully assembled large ribosomal subunit, suggesting that the methylations take place during the assembly of the ribosome. The key recognition elements in the 23S rRNA are helices 90-92 and the adjacent single stranded RNA that encompasses A2503. To our knowledge, this study represents the first in vitro description of a methyl transfer catalyzed by a member of the Radical SAM superfamily, and it expands the catalytic repertoire of this diverse enzyme class. Furthermore, by providing information on both the timing of methylation and its substrate requirements, our findings have important implications for the functional consequences of Cfr-mediated modification of rRNA in the acquisition of antibiotic resistance.
J Am Chem Soc
2010;132(11):3953-3964
| PubMed ID:
20184321
Fujimori DG
Radical SAM-mediated methylation reactions
▸ Abstract
A subset of enzymes that belong to the radical S-adenosylmethionine (SAM) superfamily is able to catalyze methylation reactions. Substrates of these enzymes are distinct from the nucleophilic substrates that undergo methylation by a polar mechanism. Recently, activities of several radical SAM methylating enzymes have been reconstituted in vitro and their mechanisms of catalysis investigated. The RNA modifying enzymes RlmN and Cfr catalyze methylation via a methyl synthase mechanism. These enzymes use SAM in two distinct roles: as a source of a methyl group transferred to a conserved cysteine and as a source of 5'-deoxyadenosyl radical (5'-dA). Hydrogen atom abstraction by this species generates a thiomethylene radical which adds into the RNA substrate, forming an enzyme-substrate covalent adduct. In another recent study, methylation of the indole moiety of tryptophan by the radical SAM and cobalamin-binding domain enzyme TsrM has been reconstituted. Methylcobalamin serves as an intermediate methyl donor in TsrM, and is proposed to transfer the methyl group as a methyl radical. Interestingly, despite the presence of the radical SAM motif, no reductive cleavage of SAM has been observed in this methylation. These important reconstitutions set the stage for further studies on mechanisms of radical methylation.
Curr Opin Chem Biol
2013;17(4):597-604
| PubMed ID:
23835516
Grove TL, Livada J, Schwalm EL, Green MT, Booker SJ, Silakov A
A substrate radical intermediate in catalysis by the antibiotic resistance protein Cfr
▸ Abstract
Cfr-dependent methylation of C8 of A2503 in 23S ribosomal RNA confers bacterial resistance to an array of clinically important antibiotics that target the large subunit of the ribosome, including the synthetic oxazolidinone antibiotic linezolid. The key element of the proposed mechanism for Cfr, a radical S-adenosylmethionine enzyme, is the addition of a methylene radical, generated by hydrogen-atom abstraction from the methyl group of an S-methylated cysteine, onto C8 of A2503 to form a protein-nucleic acid crosslinked species containing an unpaired electron. Herein we use continuous-wave and pulsed EPR techniques to provide direct spectroscopic evidence for this intermediate, showing a spin-delocalized radical with maximum spin density at N7 of the adenine ring. In addition, we use rapid freeze-quench EPR to show that the radical forms and decays with rate constants that are consistent with the rate of formation of the methylated product.
Nat Chem Biol
2013;9(7):422-427
| PubMed ID:
23644479
Grove TL, Benner JS, Radle MI, Ahlum JH, Landgraf BJ, Krebs C, Booker SJ
A radically different mechanism for S-adenosylmethionine-dependent methyltransferases
▸ Abstract
Methylation of small molecules and macromolecules is crucial in metabolism, cell signaling, and epigenetic programming and is most often achieved by S-adenosylmethionine (SAM)-dependent methyltransferases. Most employ an S(N)2 mechanism to methylate nucleophilic sites on their substrates, but recently, radical SAM enzymes have been identified that methylate carbon atoms that are not inherently nucleophilic via the intermediacy of a 5'-deoxyadenosyl 5'-radical. We have determined the mechanisms of two such reactions targeting the sp(2)-hybridized carbons at positions 2 and 8 of adenosine 2503 in 23S ribosomal RNA, catalyzed by RlmN and Cfr, respectively. In neither case is a methyl group transferred directly from SAM to the RNA; rather, both reactions proceed by a ping-pong mechanism involving intermediate methylation of a conserved cysteine residue.
Science
2011;332(6029):604-607
| PubMed ID:
21415317
Kehrenberg C, Schwarz S, Jacobsen L, Hansen LH, Vester B
A new mechanism for chloramphenicol, florfenicol and clindamycin resistance: methylation of 23S ribosomal RNA at A2503
▸ Abstract
The gene product of cfr from Staphylococcus sciuri confers resistance to chloramphenicol, florfenicol and clindamycin in Staphylococcus spp. and Escherichia coli. Cfr is not similar to any other known chloramphenicol resistance determinant. Comparative investigation of E. coli with and without a plasmid-encoded Cfr showed a decreased drug binding to ribosomes in the presence of Cfr. As chloramphenicol/florfenicol and clindamycin have partly overlapping drug binding sites on the ribosome, the most likely explanation is that Cfr modifies the RNA in the drug binding site. This hypothesis was supported by drug footprinting data that showed both a decreased drug binding and an enhanced reverse transcriptase stop at position 2504, which corresponds to a modification at position A2503 at the drug binding site. A 45 n long RNA fragment containing the appropriate region was isolated and MALDI-TOF mass spectrometry in combination with tandem mass spectrometry showed an additional methylation at position A2503. Moreover, reduced methylation was detected at nucleotide C2498. The results show that Cfr is an RNA methyltransferase that targets nucleotide A2503 and inhibits ribose methylation at nucleotide C2498, thereby causing resistance to chloramphenicol, florfenicol and clindamycin.
Mol Microbiol
2005;57(4):1064-1073
| PubMed ID:
16091044
Mendes RE, Deshpande LM, Castanheira M, DiPersio J, Saubolle MA, Jones RN
First report of cfr-mediated resistance to linezolid in human staphylococcal clinical isolates recovered in the United States
▸ Abstract
Linezolid resistance has dominantly been mediated by mutations in 23S rRNA or ribosomal protein L4 genes. Recently, cfr has demonstrated the ability to produce a phenotype of resistance to not only oxazolidinones, but also other antimicrobial classes (phenicols, lincosamides, pleuromutilins, and streptogramin A). We describe the first detection of cfr-mediated linezolid resistance in Staphylococcus aureus and Staphylococcus epidermidis recovered from human infection cases monitored during the 2007 LEADER Program.
Antimicrob Agents Chemother
2008;52(6):2244-2246
| PubMed ID:
18391032