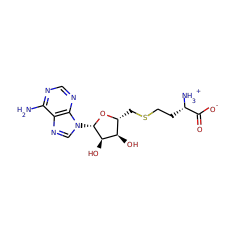
Top Level Name
⌊ Superfamily (core) Radical SAM
⌊ Subgroup methyltransferase (Class A)
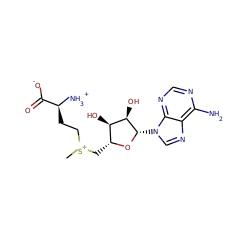
⌊ Family adenosine C2 methyltransferase (RlmN-like)
⌊ FunctionalDomain Ribosomal RNA large subunit methyltransferase N (ID 380572)
No Notes.
| Superfamily Assignment Evidence Code(s) | FSM |
| Family Assignment Evidence Code | CFM PubMed:21527678 PubMed:24806349 PubMed:18025251 PubMed:21415317 PubMed:22891362 |
| This entry was last updated on | June 10, 2017 |
References to Other Databases
Genbank
| Species | GI | Accession | Proteome |
|---|---|---|---|
| Escherichia coli str. K-12 substr. MG1655 Taxon ID: 511145 | 16130442 | NP_417012.1 (RefSeq) | URP |
| Taxon ID: 543 | 445925462 | WP_000003317.1 (RefSeq) | |
| Escherichia coli O104:H4 str. 2011C-3493 Taxon ID: 1133852 | 407480925 | YP_006778074.1 (RefSeq) | URP |
| Escherichia coli O83:H1 str. NRG 857C Taxon ID: 685038 | 387617833 | YP_006120855.1 (RefSeq) | URP |
| Escherichia coli IAI39 Taxon ID: 585057 | 218701027 | YP_002408656.1 (RefSeq) | URP |
| Shigella dysenteriae Sd197 Taxon ID: 300267 | 82777902 | YP_404251.1 (RefSeq) | URP |
| Escherichia coli Taxon ID: 562 | 857141558 | AKO58025.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 850015677 | AKN48446.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 848540703 | KMM08694.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 848537905 | KMM06164.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 848534346 | KMM03000.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 848529530 | KML98401.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 848522069 | KML91324.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 848516947 | KML86673.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 848512943 | KML82828.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 848504567 | KML75104.1 (Genbank) | URP |
| Klebsiella pneumoniae Taxon ID: 573 | 839391824 | KME68812.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 838821166 | KLY03335.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 838817239 | KLX99429.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 838813592 | KLX95814.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 838805369 | KLX87663.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 838801061 | KLX83403.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 838800350 | KLX82695.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 838792856 | KLX75297.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 838788352 | KLX70840.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 838779659 | KLX62263.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 838777935 | KLX60560.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 838775940 | KLX58582.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 838770274 | KLX53001.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 838752115 | KLX35162.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 838750832 | KLX33882.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 838745252 | KLX28328.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 838739321 | KLX22476.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 838721608 | KLX04930.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 838717955 | KLX01304.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 838715333 | KLW98702.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 836408241 | KLU93852.1 (Genbank) | URP |
| Escherichia coli PCN061 Taxon ID: 1358422 | 834765601 | AKM36073.1 (Genbank) | URP |
| Escherichia coli PCN033 Taxon ID: 1001989 | 828389428 | AKK49258.1 (Genbank) | URP |
| Escherichia coli GM4792 Taxon ID: 1411691 | 827622689 | AKK18423.1 (Genbank) | |
| Escherichia coli GM4792 Taxon ID: 1411691 | 827619539 | AKK15522.1 (Genbank) | |
| Shigella boydii Taxon ID: 621 | 827431045 | AKI67604.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 823071275 | KLH91810.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 823070514 | KLH91062.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 823064554 | KLH85243.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 823059609 | KLH80459.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 823050349 | KLH71572.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 823016290 | KLH38813.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 823009318 | KLH32018.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 823005824 | KLH28661.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 822999859 | KLH22876.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 822993817 | KLH17014.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 822990483 | KLH13745.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 822984915 | KLH08329.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 822982863 | KLH06335.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 822975952 | KLG99580.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 822968241 | KLG92133.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 822963552 | KLG87591.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 822958664 | KLG82933.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 822955283 | KLG79706.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 822946761 | KLG71493.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 822944915 | KLG69696.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 822937516 | KLG62473.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 822927144 | KLG52402.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 822925218 | KLG50528.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 822922072 | KLG47482.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 822918092 | KLG43608.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 822910030 | KLG37808.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 822898138 | KLG30747.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 822081762 | KLD53798.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 822076639 | KLD48754.1 (Genbank) | URP |
| Shigella sonnei Taxon ID: 624 | 821338280 | AKH31491.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 821330122 | AKH23225.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 817592327 | AKF72906.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 817588187 | AKF68767.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 817584046 | AKF64627.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 817579907 | AKF60489.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 817575765 | AKF56349.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 817149030 | AKF21618.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 817030222 | KKO38339.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 817025993 | KKO34284.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 817022221 | KKO30702.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 817021106 | KKO29631.1 (Genbank) | URP |
| Escherichia coli NB8 Taxon ID: 1431454 | 816243632 | KKK02045.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. C227-11 Taxon ID: 1048254 | 816209439 | AKE83245.1 (Genbank) | URP |
| Escherichia coli MRSN 10204 Taxon ID: 1409953 | 816061424 | KKJ09359.1 (Genbank) | URP |
| Escherichia coli K-12 Taxon ID: 83333 | 808735845 | AKD92680.1 (Genbank) | PRP URP |
| Escherichia coli K-12 Taxon ID: 83333 | 808731416 | AKD88252.1 (Genbank) | PRP URP |
| Escherichia coli K-12 Taxon ID: 83333 | 808723100 | AKD83896.1 (Genbank) | PRP URP |
| Escherichia coli K-12 Taxon ID: 83333 | 808718728 | AKD79525.1 (Genbank) | PRP URP |
| Escherichia coli K-12 Taxon ID: 83333 | 808714318 | AKD75116.1 (Genbank) | PRP URP |
| Escherichia coli K-12 Taxon ID: 83333 | 808709956 | AKD70755.1 (Genbank) | PRP URP |
| Escherichia coli K-12 Taxon ID: 83333 | 808705596 | AKD66396.1 (Genbank) | PRP URP |
| Escherichia coli K-12 Taxon ID: 83333 | 808701222 | AKD62023.1 (Genbank) | PRP URP |
| Escherichia coli Taxon ID: 562 | 806962051 | AKC15356.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 803480960 | KKB22150.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 803472660 | KKB14815.1 (Genbank) | URP |
| Escherichia coli 9.1649 Taxon ID: 869685 | 802471875 | KKA60003.1 (Genbank) | URP |
| Escherichia coli VR50 Taxon ID: 941323 | 801069118 | AKA91615.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 796820559 | KJY11141.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 788922705 | KJW71742.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 788918534 | KJW67983.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 788916856 | KJW66380.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 788912609 | KJW62273.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 788904206 | KJW54454.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 788893673 | KJW44444.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 788889084 | KJW40183.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 788884763 | KJW36138.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 788873671 | KJW25398.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 788870705 | KJW22658.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 768594850 | KJI28409.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 768577003 | KJI10972.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 768573581 | KJI07688.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 767819881 | KJH00495.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 767814632 | KJG95431.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 765196455 | KJD94436.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 765194366 | KJD92364.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 765184641 | KJD82934.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 765182847 | KJD81155.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 765181212 | KJD79534.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 765109752 | KJD69308.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 765104076 | KJD63742.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 763539230 | KJA05941.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 763534203 | KJA01114.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 763531173 | KIZ98243.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 763524594 | KIZ92060.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 763523149 | KIZ90695.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 763516982 | KIZ84859.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 763509200 | KIZ77598.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 763505798 | KIZ74388.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 763499780 | KIZ68770.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 763497908 | KIZ67073.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 763492506 | KIZ61914.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 758863595 | AJO84553.1 (Genbank) | URP |
| Escherichia coli RS218 Taxon ID: 439184 | 756770440 | AJM74711.1 (Genbank) | URP |
| n/a | 756612913 | AJM25260.1 (Genbank) | |
| Escherichia coli Taxon ID: 562 | 755817797 | KIQ46662.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 755718687 | KIQ40375.1 (Genbank) | URP |
| Escherichia coli 97.0264 Taxon ID: 869676 | 753925868 | KIO87692.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 753776776 | AJH11205.1 (Genbank) | URP |
| Escherichia coli E24377A Taxon ID: 331111 | 752463142 | KIO41254.1 (Genbank) | URP |
| Escherichia coli ECC-1470 Taxon ID: 758831 | 752306897 | AJG09516.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 752195614 | KIN87343.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 751401601 | AJF77686.1 (Genbank) | URP |
| Escherichia coli 1303 Taxon ID: 745156 | 751381546 | AJF57373.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 749030112 | KII11002.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 748270643 | AJE57077.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 748130462 | KIH35477.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 748121008 | KIH26383.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 748120717 | KIH26093.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 748116168 | KIH21654.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 748112578 | KIH18536.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 748106530 | KIH13036.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 748096121 | KIH03024.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 748093256 | KIH00059.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 748092894 | KIG99848.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 748086008 | KIG93182.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 748080073 | KIG87413.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 748074175 | KIG81793.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 748071982 | KIG79736.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 748064097 | KIG73168.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 748056217 | KIG66190.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 748051239 | KIG61603.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 748050081 | KIG60532.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 748043599 | KIG54603.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 748032967 | KIG44495.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 748029854 | KIG41439.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 748026508 | KIG38197.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 748020629 | KIG32687.1 (Genbank) | URP |
| Escherichia coli C691-71 (14b) Taxon ID: 1010817 | 747264117 | KIG25506.1 (Genbank) | URP |
| Escherichia coli RS218 Taxon ID: 439184 | 746690567 | KIE81688.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 746680278 | KIE71512.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 746678807 | KIE70066.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 742951962 | AJB52631.1 (Genbank) | URP |
| Escherichia coli APEC IMT5155 Taxon ID: 1329907 | 742673465 | AJB35652.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 738567535 | KHQ70982.1 (Genbank) | URP |
| Shigella flexneri Taxon ID: 623 | 738561102 | KHQ64585.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 735081156 | KHO58158.1 (Genbank) | URP |
| Escherichia coli str. K-12 substr. MG1655 Taxon ID: 511145 | 732683610 | AIZ90415.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 732681290 | AIZ88097.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 732676783 | AIZ83592.1 (Genbank) | URP |
| Escherichia coli K-12 Taxon ID: 83333 | 731471452 | AIZ52334.1 (Genbank) | PRP URP |
| Escherichia coli ER2796 Taxon ID: 1245474 | 730583723 | AIZ29009.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 730039634 | KHJ27163.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 730034403 | KHJ22036.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 730033796 | KHJ21448.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 730025994 | KHJ14058.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 730021506 | KHJ09802.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 730012500 | KHJ01166.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 730011292 | KHI99975.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 730009298 | KHI98008.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 730001156 | KHI90079.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729991789 | KHI81060.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729991037 | KHI80312.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729981705 | KHI71334.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729979376 | KHI69041.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729972912 | KHI62806.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729968375 | KHI58361.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729962233 | KHI52366.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729957816 | KHI48196.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729949736 | KHI40316.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729945694 | KHI36375.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729941326 | KHI32108.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729930046 | KHI21370.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729925853 | KHI17260.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729919514 | KHI11155.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729915314 | KHI07158.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729914457 | KHI06308.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729901807 | KHH94014.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729898355 | KHH90663.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729893726 | KHH86362.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729892286 | KHH84933.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729874685 | KHH67674.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729873444 | KHH66466.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729867013 | KHH60159.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729862840 | KHH56121.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729859133 | KHH52478.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729855858 | KHH49334.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729852814 | KHH46403.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729846105 | KHH39884.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729840832 | KHH34764.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729836279 | KHH30253.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729828464 | KHH22660.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729823365 | KHH17631.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729816576 | KHH11061.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729809345 | KHH04148.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729807089 | KHH02011.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729802913 | KHG97980.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729792421 | KHG87950.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729787214 | KHG82892.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729786832 | KHG82517.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 729777182 | KHG73120.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 724499165 | KHD58565.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 724496406 | KHD55842.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 724490696 | KHD50197.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 724483547 | KHD43271.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 723231763 | AIX64406.1 (Genbank) | URP |
| Shigella sonnei Taxon ID: 624 | 721550426 | KGY93764.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 706064320 | KGT32538.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 706059856 | KGT28468.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 706053136 | KGT22321.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 706052133 | KGT21352.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 706042961 | KGT12700.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 705993375 | KGT06798.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 701216383 | KGP55340.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 701213931 | KGP52900.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 701199995 | KGP39548.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 700828769 | KGP21307.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 700817576 | KGP11099.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 700817293 | KGP10831.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 699406333 | KGM84560.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 699404525 | KGM82810.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 699396088 | KGM74994.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 699391818 | KGM70991.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 699386514 | KGM65969.1 (Genbank) | URP |
| Escherichia coli NCTC 50110 Taxon ID: 1333537 | 697409623 | KGL70688.1 (Genbank) | URP |
| Escherichia coli FAP1 Taxon ID: 1412834 | 695939411 | AIT35642.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 693352486 | KGI49004.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 685226078 | KGA88095.1 (Genbank) | URP |
| Escherichia coli BW25113 Taxon ID: 679895 | 682119992 | AIN32918.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 678170041 | KFV38135.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 678164792 | KFV33095.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 678157952 | KFV26417.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 678156612 | KFV25101.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 672836929 | KFH81134.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 671700732 | KFF52691.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 671390761 | KFF37385.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 669335556 | KFD75529.1 (Genbank) | URP |
| Escherichia coli DSM 30083 Taxon ID: 866789 | 668709456 | KFB95247.1 (Genbank) | URP |
| Escherichia coli B7A Taxon ID: 340184 | 664697638 | AIF64291.1 (Genbank) | URP |
| Escherichia coli KLY Taxon ID: 1435461 | 664684816 | AIF37767.1 (Genbank) | URP |
| Escherichia coli 1140 Taxon ID: 1453472 | 662000654 | KEP77238.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 660678619 | KEP17983.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 660670251 | KEP09736.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 660657952 | KEO97611.1 (Genbank) | URP |
| Escherichia coli 2-460-02_S1_C2 Taxon ID: 1444073 | 660351703 | KEO38590.1 (Genbank) | URP |
| Escherichia coli 1-250-04_S3_C2 Taxon ID: 1444163 | 660344154 | KEO31255.1 (Genbank) | URP |
| Escherichia coli 2-460-02_S1_C1 Taxon ID: 1444044 | 660342756 | KEO29913.1 (Genbank) | URP |
| Escherichia coli 5-366-08_S4_C1 Taxon ID: 1444212 | 660335629 | KEO22969.1 (Genbank) | URP |
| Escherichia coli 2-222-05_S4_C3 Taxon ID: 1444264 | 660328620 | KEO16353.1 (Genbank) | URP |
| Escherichia coli 2-177-06_S3_C3 Taxon ID: 1444179 | 660321335 | KEO09213.1 (Genbank) | URP |
| Escherichia coli 8-415-05_S1_C2 Taxon ID: 1444076 | 660321124 | KEO09004.1 (Genbank) | URP |
| Escherichia coli 1-392-07_S4_C1 Taxon ID: 1444219 | 660310841 | KEN99094.1 (Genbank) | URP |
| Escherichia coli 2-222-05_S3_C1 Taxon ID: 1444123 | 660300136 | KEN89515.1 (Genbank) | URP |
| Escherichia coli 2-474-04_S4_C1 Taxon ID: 1444210 | 660294776 | KEN84297.1 (Genbank) | URP |
| Escherichia coli 2-052-05_S3_C2 Taxon ID: 1444149 | 660286511 | KEN76518.1 (Genbank) | URP |
| Escherichia coli 1-392-07_S4_C3 Taxon ID: 1444275 | 660276713 | KEN66898.1 (Genbank) | URP |
| Escherichia coli 6-537-08_S4_C2 Taxon ID: 1444257 | 660272502 | KEN62797.1 (Genbank) | URP |
| Escherichia coli 6-537-08_S1_C3 Taxon ID: 1444116 | 660264909 | KEN55377.1 (Genbank) | URP |
| Escherichia coli 7-233-03_S4_C3 Taxon ID: 1444270 | 660264309 | KEN54788.1 (Genbank) | URP |
| Escherichia coli 6-537-08_S1_C2 Taxon ID: 1444091 | 660257013 | KEN47629.1 (Genbank) | URP |
| Escherichia coli 7-233-03_S4_C1 Taxon ID: 1444213 | 660251612 | KEN42326.1 (Genbank) | URP |
| Escherichia coli 8-415-05_S1_C1 Taxon ID: 1444048 | 660233053 | KEN24414.1 (Genbank) | URP |
| Escherichia coli 7-233-03_S3_C2 Taxon ID: 1444158 | 660232787 | KEN24150.1 (Genbank) | URP |
| Escherichia coli 6-537-08_S3_C2 Taxon ID: 1444174 | 660225675 | KEN17213.1 (Genbank) | URP |
| Escherichia coli 7-233-03_S4_C2 Taxon ID: 1444241 | 660220946 | KEN12603.1 (Genbank) | URP |
| Escherichia coli 7-233-03_S3_C3 Taxon ID: 1444188 | 660211880 | KEN03760.1 (Genbank) | URP |
| Escherichia coli 6-319-05_S1_C1 Taxon ID: 1444062 | 660211663 | KEN03550.1 (Genbank) | URP |
| Escherichia coli 6-537-08_S4_C1 Taxon ID: 1444227 | 660199214 | KEM91374.1 (Genbank) | URP |
| Escherichia coli 2-222-05_S4_C1 Taxon ID: 1444206 | 660197505 | KEM89717.1 (Genbank) | URP |
| Escherichia coli 6-537-08_S3_C3 Taxon ID: 1444199 | 660191117 | KEM83550.1 (Genbank) | URP |
| Escherichia coli 6-537-08_S3_C1 Taxon ID: 1444146 | 660183244 | KEM75873.1 (Genbank) | URP |
| Escherichia coli 7-233-03_S3_C1 Taxon ID: 1444130 | 660179469 | KEM72162.1 (Genbank) | URP |
| Escherichia coli 6-319-05_S3_C3 Taxon ID: 1444198 | 660167890 | KEM60811.1 (Genbank) | URP |
| Escherichia coli 6-175-07_S1_C3 Taxon ID: 1444114 | 660158867 | KEM52127.1 (Genbank) | URP |
| Escherichia coli 6-175-07_S4_C3 Taxon ID: 1444281 | 660153898 | KEM47320.1 (Genbank) | URP |
| Escherichia coli 6-537-08_S1_C1 Taxon ID: 1444063 | 660146390 | KEM40266.1 (Genbank) | URP |
| Escherichia coli 6-319-05_S3_C2 Taxon ID: 1444173 | 660135254 | KEM29386.1 (Genbank) | URP |
| Escherichia coli 6-319-05_S1_C3 Taxon ID: 1444115 | 660132862 | KEM27033.1 (Genbank) | URP |
| Escherichia coli 6-319-05_S3_C1 Taxon ID: 1444145 | 660126300 | KEM20611.1 (Genbank) | URP |
| Escherichia coli 6-319-05_S1_C2 Taxon ID: 1444090 | 660119571 | KEM14219.1 (Genbank) | URP |
| Escherichia coli 6-175-07_S4_C1 Taxon ID: 1444226 | 660113047 | KEM07810.1 (Genbank) | URP |
| Escherichia coli 6-175-07_S4_C2 Taxon ID: 1444255 | 660111712 | KEM06495.1 (Genbank) | URP |
| Escherichia coli 6-175-07_S3_C3 Taxon ID: 1444197 | 660106296 | KEM01158.1 (Genbank) | URP |
| Escherichia coli 6-175-07_S3_C1 Taxon ID: 1444144 | 660097211 | KEL92210.1 (Genbank) | URP |
| Escherichia coli 5-366-08_S3_C1 Taxon ID: 1444129 | 660096380 | KEL91391.1 (Genbank) | URP |
| Escherichia coli 5-366-08_S3_C2 Taxon ID: 1444157 | 660090703 | KEL85824.1 (Genbank) | URP |
| Escherichia coli 5-366-08_S3_C3 Taxon ID: 1444187 | 660078062 | KEL73494.1 (Genbank) | URP |
| Escherichia coli 5-366-08_S1_C1 Taxon ID: 1444047 | 660074313 | KEL69839.1 (Genbank) | URP |
| Escherichia coli 5-172-05_S1_C3 Taxon ID: 1444101 | 660070921 | KEL66600.1 (Genbank) | URP |
| Escherichia coli 5-172-05_S4_C3 Taxon ID: 1444269 | 660067258 | KEL63012.1 (Genbank) | URP |
| Escherichia coli 5-172-05_S3_C1 Taxon ID: 1444128 | 660058485 | KEL54511.1 (Genbank) | URP |
| Escherichia coli 6-175-07_S1_C1 Taxon ID: 1444061 | 660053055 | KEL49180.1 (Genbank) | URP |
| Escherichia coli 5-172-05_S3_C3 Taxon ID: 1444186 | 660049754 | KEL45985.1 (Genbank) | URP |
| Escherichia coli 5-172-05_S4_C1 Taxon ID: 1444211 | 660041844 | KEL39628.1 (Genbank) | URP |
| Escherichia coli 5-366-08_S4_C2 Taxon ID: 1444240 | 660037724 | KEL35629.1 (Genbank) | URP |
| Escherichia coli 5-172-05_S4_C2 Taxon ID: 1444239 | 660030234 | KEL28365.1 (Genbank) | URP |
| Escherichia coli 3-373-03_S4_C3 Taxon ID: 1444279 | 660026712 | KEL24871.1 (Genbank) | URP |
| Escherichia coli 4-203-08_S3_C1 Taxon ID: 1444143 | 660019621 | KEL18129.1 (Genbank) | URP |
| Escherichia coli 4-203-08_S3_C2 Taxon ID: 1444171 | 660016517 | KEL15122.1 (Genbank) | URP |
| Escherichia coli 4-203-08_S4_C2 Taxon ID: 1444254 | 660012153 | KEL10812.1 (Genbank) | URP |
| Escherichia coli 4-203-08_S3_C3 Taxon ID: 1444196 | 660004799 | KEL03767.1 (Genbank) | URP |
| Escherichia coli 4-203-08_S1_C3 Taxon ID: 1444113 | 660001442 | KEL00462.1 (Genbank) | URP |
| Escherichia coli 4-203-08_S1_C2 Taxon ID: 1444088 | 659995422 | KEK94527.1 (Genbank) | URP |
| Escherichia coli 3-475-03_S3_C2 Taxon ID: 1444170 | 659988745 | KEK88125.1 (Genbank) | URP |
| Escherichia coli 3-475-03_S1_C2 Taxon ID: 1444087 | 659984124 | KEK83629.1 (Genbank) | URP |
| Escherichia coli 3-475-03_S3_C1 Taxon ID: 1444142 | 659978954 | KEK78553.1 (Genbank) | URP |
| Escherichia coli 6-175-07_S3_C2 Taxon ID: 1444172 | 658736418 | KEJ77254.1 (Genbank) | URP |
| Escherichia coli 5-366-08_S1_C3 Taxon ID: 1444102 | 658733451 | KEJ74358.1 (Genbank) | URP |
| Escherichia coli 3-020-07_S3_C2 Taxon ID: 1444165 | 658726150 | KEJ67440.1 (Genbank) | URP |
| Escherichia coli 3-267-03_S4_C1 Taxon ID: 1444223 | 658718585 | KEJ60222.1 (Genbank) | URP |
| Escherichia coli 2-460-02_S4_C1 Taxon ID: 1444209 | 658707322 | KEJ49461.1 (Genbank) | URP |
| Escherichia coli 2-427-07_S4_C3 Taxon ID: 1444266 | 658703704 | KEJ45999.1 (Genbank) | URP |
| Escherichia coli 2-460-02_S1_C3 Taxon ID: 1444100 | 658696721 | KEJ39428.1 (Genbank) | URP |
| Escherichia coli 6-175-07_S1_C2 Taxon ID: 1444089 | 658667645 | KEJ11836.1 (Genbank) | URP |
| Escherichia coli 2-427-07_S1_C1 Taxon ID: 1444043 | 652292091 | KDZ95294.1 (Genbank) | URP |
| Escherichia coli 3-105-05_S1_C3 Taxon ID: 1444110 | 652289464 | KDZ92800.1 (Genbank) | URP |
| Escherichia coli 3-105-05_S1_C2 Taxon ID: 1444084 | 652284668 | KDZ88349.1 (Genbank) | URP |
| Escherichia coli 3-073-06_S4_C3 Taxon ID: 1444277 | 652276716 | KDZ80668.1 (Genbank) | URP |
| Escherichia coli 3-073-06_S4_C2 Taxon ID: 1444249 | 652271719 | KDZ76146.1 (Genbank) | URP |
| Escherichia coli 3-073-06_S3_C1 Taxon ID: 1444138 | 652264949 | KDZ69889.1 (Genbank) | URP |
| Escherichia coli 3-073-06_S3_C2 Taxon ID: 1444166 | 652264580 | KDZ69532.1 (Genbank) | URP |
| Escherichia coli 3-073-06_S1_C2 Taxon ID: 1444083 | 652261096 | KDZ66134.1 (Genbank) | URP |
| Escherichia coli 3-020-07_S4_C3 Taxon ID: 1444276 | 652245664 | KDZ51843.1 (Genbank) | URP |
| Escherichia coli 3-073-06_S1_C1 Taxon ID: 1444055 | 652244427 | KDZ50634.1 (Genbank) | URP |
| Escherichia coli 3-020-07_S4_C2 Taxon ID: 1444248 | 652236257 | KDZ42834.1 (Genbank) | URP |
| Escherichia coli 3-020-07_S3_C1 Taxon ID: 1444137 | 652229741 | KDZ36584.1 (Genbank) | URP |
| Escherichia coli 3-020-07_S1_C3 Taxon ID: 1444108 | 652226032 | KDZ33134.1 (Genbank) | URP |
| Escherichia coli 3-020-07_S1_C2 Taxon ID: 1444082 | 652220177 | KDZ27747.1 (Genbank) | URP |
| Escherichia coli 3-020-07_S1_C1 Taxon ID: 1444054 | 652215801 | KDZ23548.1 (Genbank) | URP |
| Escherichia coli 2-474-04_S3_C3 Taxon ID: 1444185 | 652210502 | KDZ18521.1 (Genbank) | URP |
| Escherichia coli 2-474-04_S4_C3 Taxon ID: 1444268 | 652207311 | KDZ15540.1 (Genbank) | URP |
| Escherichia coli 2-474-04_S4_C2 Taxon ID: 1444238 | 652197998 | KDZ06743.1 (Genbank) | URP |
| Escherichia coli 2-474-04_S1_C2 Taxon ID: 1444074 | 652194433 | KDZ03284.1 (Genbank) | URP |
| Escherichia coli 2-474-04_S3_C2 Taxon ID: 1444156 | 652188355 | KDY97686.1 (Genbank) | URP |
| Escherichia coli 2-427-07_S1_C3 Taxon ID: 1444099 | 652183878 | KDY93453.1 (Genbank) | URP |
| Escherichia coli 2-474-04_S3_C1 Taxon ID: 1444127 | 652180810 | KDY90457.1 (Genbank) | URP |
| Escherichia coli 2-474-04_S1_C1 Taxon ID: 1444045 | 652171515 | KDY81916.1 (Genbank) | URP |
| Escherichia coli 2-460-02_S4_C3 Taxon ID: 1444267 | 652163055 | KDY73914.1 (Genbank) | URP |
| Escherichia coli 2-460-02_S3_C3 Taxon ID: 1444184 | 652156672 | KDY68169.1 (Genbank) | URP |
| Escherichia coli 2-460-02_S4_C2 Taxon ID: 1444237 | 652155450 | KDY66990.1 (Genbank) | URP |
| Escherichia coli 2-460-02_S3_C2 Taxon ID: 1444155 | 652147404 | KDY59256.1 (Genbank) | URP |
| Escherichia coli 2-460-02_S3_C1 Taxon ID: 1444126 | 652139016 | KDY51722.1 (Genbank) | URP |
| Escherichia coli 2-427-07_S4_C1 Taxon ID: 1444208 | 652136122 | KDY49219.1 (Genbank) | URP |
| Escherichia coli 2-427-07_S4_C2 Taxon ID: 1444236 | 652133155 | KDY46312.1 (Genbank) | URP |
| Escherichia coli 2-427-07_S3_C3 Taxon ID: 1444183 | 652121984 | KDY35895.1 (Genbank) | URP |
| Escherichia coli 2-427-07_S3_C1 Taxon ID: 1444125 | 652118804 | KDY32759.1 (Genbank) | URP |
| Escherichia coli 2-427-07_S1_C2 Taxon ID: 1444072 | 652112336 | KDY26512.1 (Genbank) | URP |
| Escherichia coli 2-316-03_S4_C2 Taxon ID: 1444235 | 652105182 | KDY19706.1 (Genbank) | URP |
| Escherichia coli 2-316-03_S4_C3 Taxon ID: 1444265 | 652103562 | KDY18119.1 (Genbank) | URP |
| Escherichia coli 2-316-03_S4_C1 Taxon ID: 1444207 | 652095786 | KDY10803.1 (Genbank) | URP |
| Escherichia coli 2-316-03_S3_C3 Taxon ID: 1444182 | 652090215 | KDY05621.1 (Genbank) | URP |
| Escherichia coli 2-316-03_S3_C2 Taxon ID: 1444154 | 652086629 | KDY02198.1 (Genbank) | URP |
| Escherichia coli 2-316-03_S3_C1 Taxon ID: 1444124 | 652082545 | KDX98355.1 (Genbank) | URP |
| Escherichia coli 2-222-05_S4_C2 Taxon ID: 1444234 | 652077269 | KDX93319.1 (Genbank) | URP |
| Escherichia coli 2-222-05_S3_C3 Taxon ID: 1444181 | 652074337 | KDX90524.1 (Genbank) | URP |
| Escherichia coli 2-222-05_S1_C3 Taxon ID: 1444098 | 652064381 | KDX81142.1 (Genbank) | URP |
| Escherichia coli 2-222-05_S1_C2 Taxon ID: 1444070 | 652057648 | KDX74738.1 (Genbank) | URP |
| Escherichia coli 2-210-07_S4_C3 Taxon ID: 1444263 | 652054082 | KDX71412.1 (Genbank) | URP |
| Escherichia coli 2-222-05_S1_C1 Taxon ID: 1444041 | 652050499 | KDX67916.1 (Genbank) | URP |
| Escherichia coli 2-210-07_S4_C2 Taxon ID: 1444233 | 652044731 | KDX62386.1 (Genbank) | URP |
| Escherichia coli 2-210-07_S3_C1 Taxon ID: 1444122 | 652040827 | KDX58803.1 (Genbank) | URP |
| Escherichia coli 2-177-06_S4_C1 Taxon ID: 1444204 | 652035214 | KDX53516.1 (Genbank) | URP |
| Escherichia coli 2-177-06_S3_C2 Taxon ID: 1444151 | 652028897 | KDX47547.1 (Genbank) | URP |
| Escherichia coli 2-156-04_S4_C3 Taxon ID: 1444261 | 652021334 | KDX40382.1 (Genbank) | URP |
| Escherichia coli 1-250-04_S1_C1 Taxon ID: 1444052 | 652008795 | KDX28552.1 (Genbank) | URP |
| Escherichia coli 1-250-04_S1_C2 Taxon ID: 1444080 | 652008025 | KDX27799.1 (Genbank) | URP |
| Escherichia coli 2-210-07_S3_C3 Taxon ID: 1444180 | 651846630 | KDX20325.1 (Genbank) | URP |
| Escherichia coli 2-177-06_S4_C3 Taxon ID: 1444262 | 651836489 | KDX10769.1 (Genbank) | URP |
| Escherichia coli 1-392-07_S3_C1 Taxon ID: 1444136 | 651831297 | KDX05738.1 (Genbank) | URP |
| Escherichia coli 2-210-07_S3_C2 Taxon ID: 1444152 | 651826044 | KDX00715.1 (Genbank) | URP |
| Escherichia coli 2-210-07_S1_C2 Taxon ID: 1444069 | 651819520 | KDW94595.1 (Genbank) | URP |
| Escherichia coli 2-210-07_S4_C1 Taxon ID: 1444205 | 651813270 | KDW88643.1 (Genbank) | URP |
| Escherichia coli 1-392-07_S1_C2 Taxon ID: 1444081 | 651808096 | KDW83683.1 (Genbank) | URP |
| Escherichia coli 2-005-03_S4_C1 Taxon ID: 1444200 | 651803344 | KDW79130.1 (Genbank) | URP |
| Escherichia coli 1-392-07_S1_C1 Taxon ID: 1444053 | 651798427 | KDW74334.1 (Genbank) | URP |
| Escherichia coli 1-392-07_S3_C2 Taxon ID: 1444164 | 651785322 | KDW61968.1 (Genbank) | URP |
| Escherichia coli 2-005-03_S3_C1 Taxon ID: 1444117 | 651784193 | KDW60910.1 (Genbank) | URP |
| Escherichia coli 2-210-07_S1_C3 Taxon ID: 1444097 | 651775920 | KDW52850.1 (Genbank) | URP |
| Escherichia coli 2-177-06_S4_C2 Taxon ID: 1444232 | 651769180 | KDW46653.1 (Genbank) | URP |
| Escherichia coli 2-177-06_S1_C3 Taxon ID: 1444096 | 651762039 | KDW39752.1 (Genbank) | URP |
| Escherichia coli 2-177-06_S1_C2 Taxon ID: 1444068 | 651754242 | KDW32316.1 (Genbank) | URP |
| Escherichia coli 2-156-04_S3_C2 Taxon ID: 1444150 | 651752779 | KDW30877.1 (Genbank) | URP |
| Escherichia coli 2-177-06_S1_C1 Taxon ID: 1444040 | 651740903 | KDW19544.1 (Genbank) | URP |
| Escherichia coli 2-156-04_S4_C1 Taxon ID: 1444203 | 651740614 | KDW19258.1 (Genbank) | URP |
| Escherichia coli 2-177-06_S3_C1 Taxon ID: 1444121 | 651737592 | KDW16310.1 (Genbank) | URP |
| Escherichia coli 2-156-04_S3_C3 Taxon ID: 1444178 | 651728284 | KDW07491.1 (Genbank) | URP |
| Escherichia coli 2-156-04_S1_C3 Taxon ID: 1444095 | 651722246 | KDW01643.1 (Genbank) | URP |
| Escherichia coli 2-156-04_S3_C1 Taxon ID: 1444120 | 651721388 | KDW00810.1 (Genbank) | URP |
| Escherichia coli 2-052-05_S4_C3 Taxon ID: 1444260 | 651705838 | KDV85708.1 (Genbank) | URP |
| Escherichia coli 2-052-05_S3_C3 Taxon ID: 1444177 | 651702406 | KDV82357.1 (Genbank) | URP |
| Escherichia coli O26:H11 str. 2011C-3274 Taxon ID: 1446729 | 651687475 | KDV69615.1 (Genbank) | URP |
| Escherichia coli O118:H16 str. 07-4255 Taxon ID: 1446547 | 651684619 | KDV66761.1 (Genbank) | URP |
| Escherichia coli O128:H2 str. 2011C-3317 Taxon ID: 1446581 | 651682023 | KDV64201.1 (Genbank) | URP |
| Escherichia coli O45:H2 str. 2010C-4211 Taxon ID: 1446744 | 651675290 | KDV57504.1 (Genbank) | URP |
| Escherichia coli O121:H19 str. 2011C-3609 Taxon ID: 1446574 | 651670515 | KDV52753.1 (Genbank) | URP |
| Escherichia coli O91:H21 str. 2009C-3740 Taxon ID: 1446765 | 651661985 | KDV44256.1 (Genbank) | URP |
| Escherichia coli O146:H21 str. 2010C-3325 Taxon ID: 1446599 | 651648552 | KDV31531.1 (Genbank) | URP |
| Escherichia coli O69:H11 str. 07-3763 Taxon ID: 1446751 | 651646973 | KDV29700.1 (Genbank) | URP |
| Escherichia coli O145:H25 str. 07-3858 Taxon ID: 1446583 | 651644889 | KDV27277.1 (Genbank) | URP |
| Escherichia coli O78:H12 str. 00-3279 Taxon ID: 1446756 | 651348462 | KDV16194.1 (Genbank) | URP |
| Escherichia coli 4-203-08_S4_C3 Taxon ID: 1444280 | 650148450 | KDU67826.1 (Genbank) | URP |
| Escherichia coli 4-203-08_S1_C1 Taxon ID: 1444060 | 650144715 | KDU64140.1 (Genbank) | URP |
| Escherichia coli 3-475-03_S4_C2 Taxon ID: 1444253 | 650139109 | KDU58671.1 (Genbank) | URP |
| Escherichia coli 3-373-03_S4_C1 Taxon ID: 1444224 | 650133008 | KDU52752.1 (Genbank) | URP |
| Escherichia coli 3-373-03_S1_C1 Taxon ID: 1444058 | 650127982 | KDU47901.1 (Genbank) | URP |
| Escherichia coli 3-373-03_S1_C3 Taxon ID: 1444112 | 650123383 | KDU43503.1 (Genbank) | URP |
| Escherichia coli 3-073-06_S4_C1 Taxon ID: 1444221 | 650115595 | KDU35856.1 (Genbank) | URP |
| Escherichia coli 3-373-03_S4_C2 Taxon ID: 1444252 | 650110304 | KDU30761.1 (Genbank) | URP |
| Escherichia coli 3-267-03_S4_C2 Taxon ID: 1444251 | 650105540 | KDU26161.1 (Genbank) | URP |
| Escherichia coli 3-267-03_S1_C1 Taxon ID: 1444057 | 650097847 | KDU18876.1 (Genbank) | URP |
| Escherichia coli 3-105-05_S3_C3 Taxon ID: 1444193 | 650094959 | KDU16196.1 (Genbank) | URP |
| Escherichia coli 3-373-03_S3_C2 Taxon ID: 1444169 | 650089504 | KDU11106.1 (Genbank) | URP |
| Escherichia coli 3-267-03_S1_C2 Taxon ID: 1444085 | 650081806 | KDU03817.1 (Genbank) | URP |
| Escherichia coli 3-267-03_S3_C2 Taxon ID: 1444168 | 650077187 | KDT99063.1 (Genbank) | URP |
| Escherichia coli 3-105-05_S4_C1 Taxon ID: 1444222 | 650072662 | KDT94631.1 (Genbank) | URP |
| Escherichia coli 3-475-03_S1_C1 Taxon ID: 1444059 | 650069401 | KDT91477.1 (Genbank) | URP |
| Escherichia coli 3-475-03_S4_C1 Taxon ID: 1444225 | 650065097 | KDT87272.1 (Genbank) | URP |
| Escherichia coli 3-373-03_S1_C2 Taxon ID: 1444086 | 650059389 | KDT81702.1 (Genbank) | URP |
| Escherichia coli 3-373-03_S3_C3 Taxon ID: 1444194 | 650054032 | KDT76582.1 (Genbank) | URP |
| Escherichia coli 3-373-03_S3_C1 Taxon ID: 1444141 | 650049033 | KDT71784.1 (Genbank) | URP |
| Escherichia coli 3-267-03_S3_C1 Taxon ID: 1444140 | 650045153 | KDT68073.1 (Genbank) | URP |
| Escherichia coli 3-267-03_S1_C3 Taxon ID: 1444111 | 650037880 | KDT60980.1 (Genbank) | URP |
| Escherichia coli 3-105-05_S4_C3 Taxon ID: 1444278 | 650033602 | KDT56863.1 (Genbank) | URP |
| Escherichia coli 3-105-05_S3_C2 Taxon ID: 1444167 | 650025580 | KDT49221.1 (Genbank) | URP |
| Escherichia coli 3-105-05_S4_C2 Taxon ID: 1444250 | 650019810 | KDT43633.1 (Genbank) | URP |
| Escherichia coli 3-105-05_S1_C1 Taxon ID: 1444056 | 650012887 | KDT37131.1 (Genbank) | URP |
| Escherichia coli 3-105-05_S3_C1 Taxon ID: 1444139 | 650012387 | KDT36639.1 (Genbank) | URP |
| Escherichia coli 2-052-05_S4_C1 Taxon ID: 1444202 | 650001991 | KDT26753.1 (Genbank) | URP |
| Escherichia coli 2-011-08_S4_C3 Taxon ID: 1444259 | 649993767 | KDT18864.1 (Genbank) | URP |
| Escherichia coli 2-052-05_S3_C1 Taxon ID: 1444119 | 649993698 | KDT18796.1 (Genbank) | URP |
| Escherichia coli 2-052-05_S1_C3 Taxon ID: 1444094 | 649986804 | KDT12074.1 (Genbank) | URP |
| Escherichia coli 2-011-08_S4_C1 Taxon ID: 1444201 | 649980140 | KDT05735.1 (Genbank) | URP |
| Escherichia coli 2-011-08_S1_C3 Taxon ID: 1444093 | 649974447 | KDT00151.1 (Genbank) | URP |
| Escherichia coli 2-011-08_S3_C1 Taxon ID: 1444118 | 649972442 | KDS98193.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 643344121 | KDP16486.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 641940264 | KDO90087.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 636875026 | KDN07996.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 636554652 | KDM85917.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 636548285 | KDM79734.1 (Genbank) | URP |
| Escherichia coli O145:H28 str. 4865/96 Taxon ID: 1078035 | 636544105 | KDM75643.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 636540808 | KDM72423.1 (Genbank) | URP |
| Escherichia coli UCI 66 Taxon ID: 1438696 | 635916024 | KDG96411.1 (Genbank) | URP |
| Escherichia coli UCI 65 Taxon ID: 1438695 | 635913530 | KDG93938.1 (Genbank) | URP |
| Escherichia coli UCI 58 Taxon ID: 1438694 | 635908022 | KDG88474.1 (Genbank) | URP |
| Escherichia coli UCI 57 Taxon ID: 1438693 | 635905783 | KDG86245.1 (Genbank) | URP |
| Escherichia coli UCI 53 Taxon ID: 1438691 | 635897538 | KDG78085.1 (Genbank) | URP |
| Escherichia coli UCI 51 Taxon ID: 1438689 | 635890186 | KDG70782.1 (Genbank) | URP |
| Escherichia coli MGH 57 Taxon ID: 1438687 | 635883031 | KDG63715.1 (Genbank) | URP |
| Escherichia coli CHS 69 Taxon ID: 1438685 | 635881025 | KDG61730.1 (Genbank) | URP |
| Escherichia coli CHS 77 Taxon ID: 1438686 | 635875810 | KDG56579.1 (Genbank) | URP |
| Escherichia coli CHS 68 Taxon ID: 1438684 | 635869266 | KDG50080.1 (Genbank) | URP |
| Escherichia coli BIDMC 79 Taxon ID: 1438683 | 635864596 | KDG45456.1 (Genbank) | URP |
| Escherichia coli BIDMC 77 Taxon ID: 1438681 | 635862106 | KDG42987.1 (Genbank) | URP |
| Escherichia coli BIDMC 78 Taxon ID: 1438682 | 635859101 | KDG39992.1 (Genbank) | URP |
| Escherichia coli BIDMC 76 Taxon ID: 1438680 | 635847158 | KDG28145.1 (Genbank) | URP |
| Escherichia coli BIDMC 75 Taxon ID: 1438679 | 635846627 | KDG27615.1 (Genbank) | URP |
| Escherichia coli BIDMC 74 Taxon ID: 1438678 | 635840307 | KDG21365.1 (Genbank) | URP |
| Escherichia coli BIDMC 73 Taxon ID: 1438677 | 635836822 | KDG17907.1 (Genbank) | URP |
| Escherichia coli BIDMC 72 Taxon ID: 1438676 | 635833310 | KDG14412.1 (Genbank) | URP |
| Escherichia coli BIDMC 71 Taxon ID: 1438675 | 635825080 | KDG06266.1 (Genbank) | URP |
| Escherichia coli BIDMC 65 Taxon ID: 1438673 | 635820727 | KDG01954.1 (Genbank) | URP |
| Escherichia coli BIDMC 70 Taxon ID: 1438674 | 635817849 | KDF99081.1 (Genbank) | URP |
| Escherichia coli BIDMC 64 Taxon ID: 1438672 | 635811072 | KDF92364.1 (Genbank) | URP |
| Escherichia coli BIDMC 63 Taxon ID: 1438671 | 635805611 | KDF86911.1 (Genbank) | URP |
| Escherichia coli BIDMC 62 Taxon ID: 1438670 | 635804641 | KDF85943.1 (Genbank) | URP |
| Escherichia coli BIDMC 58 Taxon ID: 1438668 | 635791465 | KDF72829.1 (Genbank) | URP |
| Escherichia coli BIDMC 59 Taxon ID: 1438669 | 635786219 | KDF67602.1 (Genbank) | URP |
| Escherichia coli 1-176-05_S4_C2 Taxon ID: 1444244 | 632196887 | KDA89248.1 (Genbank) | URP |
| Escherichia coli 2-011-08_S3_C3 Taxon ID: 1444176 | 632190913 | KDA83989.1 (Genbank) | URP |
| Escherichia coli 2-011-08_S3_C2 Taxon ID: 1444148 | 632184975 | KDA78856.1 (Genbank) | URP |
| Escherichia coli 1-182-04_S1_C2 Taxon ID: 1444079 | 632172359 | KDA67912.1 (Genbank) | URP |
| Escherichia coli 2-052-05_S1_C1 Taxon ID: 1444038 | 632166920 | KDA62967.1 (Genbank) | URP |
| Escherichia coli 2-011-08_S1_C1 Taxon ID: 1444037 | 632160283 | KDA57209.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 629842879 | KCW96880.1 (Genbank) | URP |
| Escherichia coli O145:H28 str. RM12581 Taxon ID: 1248823 | 628078308 | AHY71834.1 (Genbank) | URP |
| Escherichia coli O145:H28 str. RM12761 Taxon ID: 1248903 | 628029710 | AHY66177.1 (Genbank) | URP |
| Escherichia coli BIDMC 82 Taxon ID: 1445862 | 612277535 | EZQ70936.1 (Genbank) | URP |
| Escherichia coli BIDMC 83 Taxon ID: 1445863 | 612268090 | EZQ61624.1 (Genbank) | URP |
| Escherichia coli O26:H1 str. 2009C-4747 Taxon ID: 1446715 | 612237697 | EZQ32115.1 (Genbank) | URP |
| Escherichia coli 2-005-03_S1_C1 Taxon ID: 1444036 | 611143823 | EZK42792.1 (Genbank) | URP |
| Escherichia coli 2-005-03_S1_C2 Taxon ID: 1444064 | 611133394 | EZK32657.1 (Genbank) | URP |
| Escherichia coli 1-182-04_S1_C1 Taxon ID: 1444051 | 611130521 | EZK29843.1 (Genbank) | URP |
| Escherichia coli 2-011-08_S1_C2 Taxon ID: 1444065 | 611121568 | EZK21208.1 (Genbank) | URP |
| Escherichia coli 1-176-05_S1_C2 Taxon ID: 1444078 | 611118801 | EZK18530.1 (Genbank) | URP |
| Escherichia coli 2-005-03_S1_C3 Taxon ID: 1444092 | 611113497 | EZK13380.1 (Genbank) | URP |
| Escherichia coli 1-176-05_S1_C3 Taxon ID: 1444105 | 611106965 | EZK07284.1 (Genbank) | URP |
| Escherichia coli 1-250-04_S1_C3 Taxon ID: 1444107 | 611096267 | EZJ97265.1 (Genbank) | URP |
| Escherichia coli 1-182-04_S1_C3 Taxon ID: 1444106 | 611094190 | EZJ95246.1 (Genbank) | URP |
| Escherichia coli 1-182-04_S3_C1 Taxon ID: 1444134 | 611091617 | EZJ92727.1 (Genbank) | URP |
| Escherichia coli 1-250-04_S3_C1 Taxon ID: 1444135 | 611082867 | EZJ84435.1 (Genbank) | URP |
| Escherichia coli 1-182-04_S3_C2 Taxon ID: 1444162 | 611081818 | EZJ83449.1 (Genbank) | URP |
| Escherichia coli 1-182-04_S3_C3 Taxon ID: 1444191 | 611075710 | EZJ77778.1 (Genbank) | URP |
| Escherichia coli 1-392-07_S3_C3 Taxon ID: 1444192 | 611068001 | EZJ70496.1 (Genbank) | URP |
| Escherichia coli 1-176-05_S4_C1 Taxon ID: 1444216 | 611066701 | EZJ69235.1 (Genbank) | URP |
| Escherichia coli 1-182-04_S4_C1 Taxon ID: 1444217 | 611054815 | EZJ58134.1 (Genbank) | URP |
| Escherichia coli 1-250-04_S4_C1 Taxon ID: 1444218 | 611050302 | EZJ53863.1 (Genbank) | URP |
| Escherichia coli 2-005-03_S4_C2 Taxon ID: 1444228 | 611046211 | EZJ49915.1 (Genbank) | URP |
| Escherichia coli 1-182-04_S4_C2 Taxon ID: 1444245 | 611036798 | EZJ40798.1 (Genbank) | URP |
| Escherichia coli 2-005-03_S4_C3 Taxon ID: 1444258 | 611034511 | EZJ38553.1 (Genbank) | URP |
| Escherichia coli 1-250-04_S4_C2 Taxon ID: 1444246 | 611033603 | EZJ37672.1 (Genbank) | URP |
| Escherichia coli 1-392-07_S4_C2 Taxon ID: 1444247 | 611023798 | EZJ28349.1 (Genbank) | URP |
| Escherichia coli 1-176-05_S4_C3 Taxon ID: 1444273 | 611017603 | EZJ22369.1 (Genbank) | URP |
| Escherichia coli 1-182-04_S4_C3 Taxon ID: 1444274 | 611014398 | EZJ19240.1 (Genbank) | URP |
| Escherichia coli O26:H1 str. 2010C-3902 Taxon ID: 1446721 | 608770702 | EZH56823.1 (Genbank) | URP |
| Escherichia coli O26:H1 str. 2010C-4244 Taxon ID: 1446722 | 608770275 | EZH56409.1 (Genbank) | URP |
| Escherichia coli O26:H1 str. 2010C-3871 Taxon ID: 1446720 | 608762286 | EZH49046.1 (Genbank) | URP |
| Escherichia coli O26:H1 str. 2010C-3472 Taxon ID: 1446719 | 608756900 | EZH43976.1 (Genbank) | URP |
| Escherichia coli O26:H1 str. 2010C-3051 Taxon ID: 1446718 | 608753585 | EZH40828.1 (Genbank) | URP |
| Escherichia coli O26:H1 str. 2009C-4760 Taxon ID: 1446716 | 608745042 | EZH33181.1 (Genbank) | URP |
| Escherichia coli O26:H1 str. 2009C-4826 Taxon ID: 1446717 | 608741806 | EZH30075.1 (Genbank) | URP |
| Escherichia coli O26:H1 str. 2009C-3996 Taxon ID: 1446714 | 608735218 | EZH23753.1 (Genbank) | URP |
| Escherichia coli O26:H1 str. 2009C-3612 Taxon ID: 1446712 | 608725318 | EZH14822.1 (Genbank) | URP |
| Escherichia coli O26:H1 str. 2009C-3689 Taxon ID: 1446713 | 608723658 | EZH13208.1 (Genbank) | URP |
| Escherichia coli O26:H1 str. 2011C-3655 Taxon ID: 1446733 | 608718052 | EZH07806.1 (Genbank) | URP |
| Escherichia coli O26:H1 str. 2011C-3506 Taxon ID: 1446732 | 608711404 | EZH01875.1 (Genbank) | URP |
| Escherichia coli O26:H1 str. 2011C-3387 Taxon ID: 1446731 | 608709401 | EZG99954.1 (Genbank) | URP |
| Escherichia coli O26:H1 str. 2011C-3282 Taxon ID: 1446730 | 608708925 | EZG99488.1 (Genbank) | URP |
| Escherichia coli O26:H1 str. 2011C-3270 Taxon ID: 1446728 | 608695134 | EZG86632.1 (Genbank) | URP |
| Escherichia coli O26:H1 str. 2010EL-1699 Taxon ID: 1446727 | 608694902 | EZG86427.1 (Genbank) | URP |
| Escherichia coli O26:H1 str. 2010C-5028 Taxon ID: 1446726 | 608683445 | EZG76073.1 (Genbank) | URP |
| Escherichia coli O26:H1 str. 2010C-4834 Taxon ID: 1446725 | 608681695 | EZG74384.1 (Genbank) | URP |
| Escherichia coli O26:H1 str. 2010C-4819 Taxon ID: 1446724 | 608672418 | EZG66099.1 (Genbank) | URP |
| Escherichia coli O26:H1 str. 2010C-4430 Taxon ID: 1446723 | 608669851 | EZG63705.1 (Genbank) | URP |
| Escherichia coli O26:H1 str. 03-3500 Taxon ID: 1446710 | 608659693 | EZG54554.1 (Genbank) | URP |
| Escherichia coli O26:H1 str. 06-3464 Taxon ID: 1446711 | 608652815 | EZG47966.1 (Genbank) | URP |
| Escherichia coli 1728 Taxon ID: 1457300 | 608092340 | EZG31398.1 (Genbank) | URP |
| Escherichia coli O121:H19 str. 2010C-3794 Taxon ID: 1446561 | 607820049 | EZE99076.1 (Genbank) | URP |
| Escherichia coli O145:NM str. 2010C-3508 Taxon ID: 1446588 | 607813496 | EZE92885.1 (Genbank) | URP |
| Escherichia coli O121:H19 str. 2009EL1302 Taxon ID: 1446558 | 607806273 | EZE85991.1 (Genbank) | URP |
| Escherichia coli O121:H19 str. 2009C-4750 Taxon ID: 1446557 | 607793320 | EZE73382.1 (Genbank) | URP |
| Escherichia coli O91:H21 str. 2009C-4646 Taxon ID: 1446766 | 607787007 | EZE67264.1 (Genbank) | URP |
| Escherichia coli O45:H2 str. 2009C-4780 Taxon ID: 1446742 | 607783106 | EZE63461.1 (Genbank) | URP |
| Escherichia coli O118:H16 str. 2009C-4446 Taxon ID: 1446549 | 607773471 | EZE54112.1 (Genbank) | URP |
| Escherichia coli O121:H19 str. 2009C-4050 Taxon ID: 1446555 | 607765299 | EZE46245.1 (Genbank) | URP |
| Escherichia coli O91:NM str. 2009C-3745 Taxon ID: 1446768 | 607755927 | EZE37203.1 (Genbank) | URP |
| Escherichia coli O123:H11 str. 2009C-3307 Taxon ID: 1446580 | 607750731 | EZE32298.1 (Genbank) | URP |
| Escherichia coli O45:H2 str. 2009C-3686 Taxon ID: 1446741 | 607742545 | EZE24306.1 (Genbank) | URP |
| Escherichia coli O69:H11 str. 2009C-3601 Taxon ID: 1446754 | 607739434 | EZE21257.1 (Genbank) | URP |
| Escherichia coli O121:H7 str. 2009C-3299 Taxon ID: 1446578 | 607732869 | EZE15253.1 (Genbank) | URP |
| Escherichia coli O69:H11 str. 08-4661 Taxon ID: 1446753 | 607731802 | EZE14208.1 (Genbank) | URP |
| Escherichia coli O103:H2 str. 2009C-3279 Taxon ID: 1446503 | 607719869 | EZE02441.1 (Genbank) | URP |
| Escherichia coli O145:H28 str. 2009C-3292 Taxon ID: 1446584 | 607715425 | EZD98133.1 (Genbank) | URP |
| Escherichia coli O91:H14 str. 2009C-3227 Taxon ID: 1446764 | 607712131 | EZD94988.1 (Genbank) | URP |
| Escherichia coli O121:H19 str. K5269 Taxon ID: 1446576 | 607567352 | EZC54585.1 (Genbank) | URP |
| Escherichia coli O121:H19 str. K5198 Taxon ID: 1446575 | 607564710 | EZC51999.1 (Genbank) | URP |
| Escherichia coli O15:H18 str. K1516 Taxon ID: 1446601 | 607464908 | EZB54516.1 (Genbank) | URP |
| Escherichia coli O169:H41 str. F9792 Taxon ID: 1446702 | 607434316 | EZB24854.1 (Genbank) | URP |
| Escherichia coli O121:H19 str. F6714 Taxon ID: 1446499 | 607402802 | EZA94534.1 (Genbank) | URP |
| Escherichia coli O6:H16 str. F5656C1 Taxon ID: 1446748 | 607383000 | EZA75439.1 (Genbank) | URP |
| Escherichia coli O25:NM str. E2539C1 Taxon ID: 1446708 | 607381137 | EZA73611.1 (Genbank) | URP |
| Escherichia coli O157:H16 str. 98-3133 Taxon ID: 1446608 | 607377963 | EZA70498.1 (Genbank) | URP |
| Escherichia coli O104:H21 str. 94-3025 Taxon ID: 1446497 | 607374789 | EZA67604.1 (Genbank) | URP |
| Escherichia coli O26:H11 str. 05-3646 Taxon ID: 1446500 | 607016369 | EZA44511.1 (Genbank) | URP |
| Escherichia coli O103:H11 str. 04-3023 Taxon ID: 1446501 | 607004286 | EZA32893.1 (Genbank) | URP |
| Escherichia coli O174:H8 str. 04-3038 Taxon ID: 1446704 | 607001274 | EZA30052.1 (Genbank) | URP |
| Escherichia coli O45:H2 str. 01-3147 Taxon ID: 1446740 | 606999134 | EZA27981.1 (Genbank) | URP |
| Escherichia coli O113:H21 str. 07-4224 Taxon ID: 1446543 | 606990389 | EZA19617.1 (Genbank) | URP |
| Escherichia coli O81:NM str. 02-3012 Taxon ID: 1446761 | 606988603 | EZA17926.1 (Genbank) | URP |
| Escherichia coli O28ac:NM str. 02-3404 Taxon ID: 1446737 | 606987683 | EZA17022.1 (Genbank) | URP |
| Escherichia coli O121:H19 str. 03-3227 Taxon ID: 1446552 | 606979928 | EZA09813.1 (Genbank) | URP |
| Escherichia coli O174:H21 str. 03-3269 Taxon ID: 1446703 | 606972467 | EZA02932.1 (Genbank) | URP |
| Escherichia coli O119:H4 str. 03-3458 Taxon ID: 1446551 | 606967324 | EYZ97939.1 (Genbank) | URP |
| Escherichia coli O118:H16 str. 06-3256 Taxon ID: 1446545 | 606958813 | EYZ89829.1 (Genbank) | URP |
| Escherichia coli O121:H19 str. 06-3003 Taxon ID: 1446553 | 606951120 | EYZ82342.1 (Genbank) | URP |
| Escherichia coli O145:NM str. 06-3484 Taxon ID: 1446585 | 606944176 | EYZ76120.1 (Genbank) | URP |
| Escherichia coli O118:H16 str. 06-3612 Taxon ID: 1446546 | 606936378 | EYZ68723.1 (Genbank) | URP |
| Escherichia coli O69:H11 str. 06-3325 Taxon ID: 1446750 | 606934516 | EYZ66946.1 (Genbank) | URP |
| Escherichia coli O79:H7 str. 06-3501 Taxon ID: 1446760 | 606928436 | EYZ61158.1 (Genbank) | URP |
| Escherichia coli O121:H19 str. 06-3822 Taxon ID: 1446554 | 606915187 | EYZ48606.1 (Genbank) | URP |
| Escherichia coli O103:H25 str. 2010C-4529 Taxon ID: 1446506 | 606888718 | EYZ23566.1 (Genbank) | URP |
| Escherichia coli O103:H2 str. 2010C-4433 Taxon ID: 1446504 | 606887146 | EYZ22028.1 (Genbank) | URP |
| Escherichia coli O145:NM str. 2010C-4557C2 Taxon ID: 1446597 | 606879194 | EYZ14367.1 (Genbank) | URP |
| Escherichia coli O121:H19 str. 2010C-4732 Taxon ID: 1446564 | 606852484 | EYY88823.1 (Genbank) | URP |
| Escherichia coli O26:NM str. 2010C-4788 Taxon ID: 1446735 | 606842909 | EYY79927.1 (Genbank) | URP |
| Escherichia coli O121:H19 str. 2010C-4824 Taxon ID: 1446565 | 606819769 | EYY58178.1 (Genbank) | URP |
| Escherichia coli O165:H25 str. 2010C-4874 Taxon ID: 1446700 | 606817275 | EYY55902.1 (Genbank) | URP |
| Escherichia coli O121:H19 str. 2010C-4966 Taxon ID: 1446566 | 606813357 | EYY52251.1 (Genbank) | URP |
| Escherichia coli O153:H2 str. 2010C-5034 Taxon ID: 1446603 | 606801579 | EYY40914.1 (Genbank) | URP |
| Escherichia coli O121:H19 str. 2010C-4989 Taxon ID: 1446567 | 606797422 | EYY37056.1 (Genbank) | URP |
| Escherichia coli O121:H19 str. 2010EL1058 Taxon ID: 1446568 | 606790840 | EYY30840.1 (Genbank) | URP |
| Escherichia coli O121:H19 str. 2011C-3072 Taxon ID: 1446569 | 606788131 | EYY28231.1 (Genbank) | URP |
| Escherichia coli O121:H19 str. 2011C-3108 Taxon ID: 1446570 | 606783487 | EYY23783.1 (Genbank) | URP |
| Escherichia coli O121:H19 str. 2011C-3216 Taxon ID: 1446571 | 606773416 | EYY14373.1 (Genbank) | URP |
| Escherichia coli O121:H19 str. 2011C-3500 Taxon ID: 1446572 | 606757917 | EYX99729.1 (Genbank) | URP |
| Escherichia coli O121:H19 str. 2011C-3537 Taxon ID: 1446573 | 606756090 | EYX97989.1 (Genbank) | URP |
| Escherichia coli O103:H2 str. 2011C-3750 Taxon ID: 1446505 | 606747586 | EYX90057.1 (Genbank) | URP |
| Escherichia coli O156:H25 str. 2011C-3602 Taxon ID: 1446605 | 606744723 | EYX87316.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. 2011EL-1675A Taxon ID: 1446507 | 606722546 | EYX66488.1 (Genbank) | URP |
| Escherichia coli O118:H16 str. 08-3651 Taxon ID: 1446548 | 606658968 | EYX06202.1 (Genbank) | URP |
| Escherichia coli O145:NM str. 08-4270 Taxon ID: 1446586 | 606655315 | EYX02811.1 (Genbank) | URP |
| Escherichia coli O86:H34 str. 99-3124 Taxon ID: 1446762 | 606543218 | EYV96866.1 (Genbank) | URP |
| Escherichia coli O6:H16 str. 99-3165 Taxon ID: 1446747 | 606539231 | EYV93024.1 (Genbank) | URP |
| Escherichia coli O121:H19 str. 2009C-4659 Taxon ID: 1446556 | 606527083 | EYV81660.1 (Genbank) | URP |
| Escherichia coli O121:H19 str. 2009EL1412 Taxon ID: 1446559 | 606524159 | EYV78824.1 (Genbank) | URP |
| Escherichia coli O145:NM str. 2010C-3507 Taxon ID: 1446587 | 606501956 | EYV57620.1 (Genbank) | URP |
| Escherichia coli O103:H11 str. 2010C-3214 Taxon ID: 1446502 | 606497100 | EYV52977.1 (Genbank) | URP |
| Escherichia coli O145:NM str. 2010C-3511 Taxon ID: 1446591 | 606491516 | EYV47564.1 (Genbank) | URP |
| Escherichia coli O145:NM str. 2010C-3509 Taxon ID: 1446589 | 606490450 | EYV46529.1 (Genbank) | URP |
| Escherichia coli O145:NM str. 2010C-3510 Taxon ID: 1446590 | 606484285 | EYV40537.1 (Genbank) | URP |
| Escherichia coli O145:NM str. 2010C-3517 Taxon ID: 1446593 | 606476485 | EYV33151.1 (Genbank) | URP |
| Escherichia coli O145:NM str. 2010C-3516 Taxon ID: 1446592 | 606474524 | EYV31276.1 (Genbank) | URP |
| Escherichia coli O145:NM str. 2010C-3518 Taxon ID: 1446594 | 606470784 | EYV27738.1 (Genbank) | URP |
| Escherichia coli O145:NM str. 2010C-3521 Taxon ID: 1446595 | 606460760 | EYV18050.1 (Genbank) | URP |
| Escherichia coli O121:H19 str. 2010C-3609 Taxon ID: 1446560 | 606456867 | EYV14260.1 (Genbank) | URP |
| Escherichia coli O145:NM str. 2010C-3526 Taxon ID: 1446596 | 606456249 | EYV13651.1 (Genbank) | URP |
| Escherichia coli O121:H19 str. 2010C-3840 Taxon ID: 1446562 | 606449605 | EYV07255.1 (Genbank) | URP |
| Escherichia coli O45:H2 str. 2010C-3876 Taxon ID: 1446743 | 606429937 | EYU88537.1 (Genbank) | URP |
| Escherichia coli O26:NM str. 2010C-4347 Taxon ID: 1446734 | 606424526 | EYU83578.1 (Genbank) | URP |
| Escherichia coli O121:H19 str. 2010C-4254 Taxon ID: 1446563 | 606422737 | EYU81887.1 (Genbank) | URP |
| Escherichia coli K02 Taxon ID: 1409786 | 601469418 | EYT11551.1 (Genbank) | URP |
| Escherichia coli 1-110-08_S1_C2 Taxon ID: 1444077 | 598733240 | EYE36335.1 (Genbank) | URP |
| Escherichia coli 1-110-08_S1_C1 Taxon ID: 1444049 | 598731909 | EYE35082.1 (Genbank) | URP |
| Escherichia coli 1-110-08_S3_C1 Taxon ID: 1444132 | 598719157 | EYE23349.1 (Genbank) | URP |
| Escherichia coli 1-110-08_S1_C3 Taxon ID: 1444104 | 598718485 | EYE22718.1 (Genbank) | URP |
| Escherichia coli 1-110-08_S3_C2 Taxon ID: 1444160 | 598716066 | EYE20388.1 (Genbank) | URP |
| Escherichia coli 1-110-08_S3_C3 Taxon ID: 1444190 | 598706792 | EYE11945.1 (Genbank) | URP |
| Escherichia coli 1-110-08_S4_C1 Taxon ID: 1444215 | 598695640 | EYE02253.1 (Genbank) | URP |
| Escherichia coli 1-110-08_S4_C2 Taxon ID: 1444243 | 598692335 | EYD99067.1 (Genbank) | URP |
| Escherichia coli 1-110-08_S4_C3 Taxon ID: 1444272 | 598691050 | EYD97840.1 (Genbank) | URP |
| Escherichia coli 1-176-05_S3_C1 Taxon ID: 1444133 | 598678502 | EYD86467.1 (Genbank) | URP |
| Escherichia coli 1-176-05_S1_C1 Taxon ID: 1444050 | 598675810 | EYD83867.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 597609235 | EYB55275.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 597609098 | EYB55139.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 597599867 | EYB46129.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 595610173 | AHM53819.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 595605723 | AHM49370.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 595601111 | AHM44761.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 595594316 | AHM38211.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 595589718 | AHM33614.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 595586817 | AHM31159.1 (Genbank) | URP |
| Escherichia coli MP1 Taxon ID: 1314835 | 587645427 | EWY52421.1 (Genbank) | URP |
| Escherichia coli EC096/10 Taxon ID: 1280937 | 582994131 | EWC54808.1 (Genbank) | URP |
| Escherichia coli BIDMC 6 Taxon ID: 1328438 | 575667101 | ETY65536.1 (Genbank) | URP |
| Escherichia coli BIDMC 49a Taxon ID: 1400158 | 575660859 | ETY59385.1 (Genbank) | URP |
| Escherichia coli BIDMC 49b Taxon ID: 1400159 | 575657891 | ETY56447.1 (Genbank) | URP |
| Escherichia coli BWH 34 Taxon ID: 1328433 | 575647268 | ETY45946.1 (Genbank) | URP |
| Escherichia coli BWH 40 Taxon ID: 1328434 | 575643927 | ETY42618.1 (Genbank) | URP |
| Escherichia coli BIDMC 2B Taxon ID: 1328436 | 575639604 | ETY38375.1 (Genbank) | URP |
| Escherichia coli BIDMC 3 Taxon ID: 1328437 | 575634491 | ETY33318.1 (Genbank) | URP |
| Escherichia coli BIDMC 15 Taxon ID: 1328440 | 575627431 | ETY26351.1 (Genbank) | URP |
| Escherichia coli BIDMC 9 Taxon ID: 1328439 | 575625748 | ETY24680.1 (Genbank) | URP |
| Escherichia coli BIDMC 17A Taxon ID: 1328441 | 575617607 | ETY16641.1 (Genbank) | URP |
| Escherichia coli BIDMC 17B Taxon ID: 1328442 | 575613107 | ETY12188.1 (Genbank) | URP |
| Escherichia coli BIDMC 19A Taxon ID: 1328443 | 575605808 | ETY04948.1 (Genbank) | URP |
| Escherichia coli BIDMC 19B Taxon ID: 1328444 | 575603499 | ETY02305.1 (Genbank) | URP |
| Escherichia coli BIDMC 20A Taxon ID: 1328446 | 575595040 | ETX94299.1 (Genbank) | URP |
| Escherichia coli BIDMC 20B Taxon ID: 1328447 | 575590735 | ETX90029.1 (Genbank) | URP |
| Escherichia coli BIDMC 43a Taxon ID: 1400156 | 575585360 | ETX84712.1 (Genbank) | URP |
| Escherichia coli BIDMC 43b Taxon ID: 1400157 | 575580842 | ETX80251.1 (Genbank) | URP |
| Escherichia coli O145:H28 str. RM13514 Taxon ID: 1248902 | 573969750 | AHG10015.1 (Genbank) | URP |
| Escherichia coli O145:H28 str. RM13516 Taxon ID: 1248915 | 573936044 | AHG15849.1 (Genbank) | URP |
| Escherichia coli O6:H16:CFA/II str. B2C Taxon ID: 1370107 | 572730064 | ETS28649.1 (Genbank) | URP |
| Escherichia coli ATCC 35150 Taxon ID: 1389955 | 567037358 | ETJ69190.1 (Genbank) | URP |
| Escherichia coli ATCC BAA-2193 Taxon ID: 1404262 | 566658967 | ETJ60859.1 (Genbank) | URP |
| human gut metagenome Taxon ID: 408170 | 566274757 | ETJ19421.1 (Genbank) | |
| Escherichia coli ATCC BAA-2196 Taxon ID: 1405292 | 566117301 | ETI75700.1 (Genbank) | URP |
| Escherichia coli ATCC BAA-2219 Taxon ID: 1405295 | 566116051 | ETI74716.1 (Genbank) | URP |
| Escherichia coli UMEA 3489-1 Taxon ID: 1281250 | 565523571 | ETF35397.1 (Genbank) | URP |
| Escherichia coli HVH 214 (4-3062198) Taxon ID: 1281141 | 565519488 | ETF31335.1 (Genbank) | URP |
| Escherichia coli HVH 177 (4-2876612) Taxon ID: 1281106 | 565505969 | ETF17868.1 (Genbank) | URP |
| Escherichia coli LAU-EC7 Taxon ID: 1389418 | 564940422 | ETE35079.1 (Genbank) | URP |
| Escherichia coli LAU-EC9 Taxon ID: 1389420 | 564939685 | ETE34356.1 (Genbank) | URP |
| Escherichia coli LAU-EC10 Taxon ID: 1417796 | 564928846 | ETE23838.1 (Genbank) | URP |
| Escherichia coli LAU-EC8 Taxon ID: 1389419 | 564905855 | ETE06259.1 (Genbank) | URP |
| Escherichia coli ATCC BAA-2215 Taxon ID: 1405294 | 564839648 | ETD51209.1 (Genbank) | URP |
| Escherichia coli E1777 Taxon ID: 1331061 | 559788128 | ESV05181.1 (Genbank) | URP |
| Shigella dysenteriae WRSd5 Taxon ID: 1401259 | 559660130 | ESU82003.1 (Genbank) | URP |
| Shigella dysenteriae WRSd3 Taxon ID: 1401327 | 559657931 | ESU80116.1 (Genbank) | URP |
| Escherichia coli ECA-727 Taxon ID: 758830 | 558724560 | EST75458.1 (Genbank) | URP |
| Escherichia coli ECA-0157 Taxon ID: 758829 | 558724333 | EST75257.1 (Genbank) | URP |
| Escherichia coli P4-NR Taxon ID: 1117111 | 558723146 | EST74229.1 (Genbank) | URP |
| Escherichia coli P4-96 Taxon ID: 1097579 | 558721711 | EST72827.1 (Genbank) | URP |
| Escherichia coli ECC-Z Taxon ID: 758832 | 558710284 | EST61601.1 (Genbank) | URP |
| Shigella dysenteriae 1617 Taxon ID: 754093 | 558574343 | AHA66481.1 (Genbank) | URP |
| Escherichia coli CE418 Taxon ID: 1331063 | 557937109 | EST00229.1 (Genbank) | URP |
| Escherichia coli CE549 Taxon ID: 1331065 | 557933543 | ESS97074.1 (Genbank) | URP |
| Escherichia coli UMEA 3148-1 Taxon ID: 1281192 | 556157201 | ESP45303.1 (Genbank) | URP |
| Escherichia coli HVH 108 (4-6924867) Taxon ID: 1281044 | 556155605 | ESP43716.1 (Genbank) | URP |
| Escherichia coli HVH 152 (4-3447545) Taxon ID: 1281085 | 556149482 | ESP37699.1 (Genbank) | URP |
| Escherichia coli HVH 178 (4-3189163) Taxon ID: 1281107 | 556143099 | ESP31394.1 (Genbank) | URP |
| Escherichia coli HVH 148 (4-3192490) Taxon ID: 1281081 | 556139270 | ESP27611.1 (Genbank) | URP |
| Escherichia coli HVH 12 (4-7653042) Taxon ID: 1280965 | 556129029 | ESP17516.1 (Genbank) | URP |
| Escherichia coli HVH 136 (4-5970458) Taxon ID: 1281069 | 556128342 | ESP16840.1 (Genbank) | URP |
| Escherichia coli HVH 36 (4-5675286) Taxon ID: 1280986 | 556119299 | ESP07916.1 (Genbank) | URP |
| Escherichia coli BWH 32 Taxon ID: 1328432 | 555092997 | ESM36120.1 (Genbank) | URP |
| Escherichia coli BIDMC 38 Taxon ID: 1328449 | 554993922 | ESL38494.1 (Genbank) | URP |
| Escherichia coli BIDMC 37 Taxon ID: 1328448 | 554993416 | ESL37989.1 (Genbank) | URP |
| Escherichia coli BIDMC 39 Taxon ID: 1328450 | 554975482 | ESL20176.1 (Genbank) | URP |
| Escherichia coli UMEA 3323-1 Taxon ID: 1281241 | 554729621 | ESK34846.1 (Genbank) | URP |
| Escherichia coli UMEA 3693-1 Taxon ID: 1281263 | 554722701 | ESK27999.1 (Genbank) | URP |
| Escherichia coli UMEA 3290-1 Taxon ID: 1281234 | 554711986 | ESK17391.1 (Genbank) | URP |
| Escherichia coli UMEA 3426-1 Taxon ID: 1281249 | 554710903 | ESK16319.1 (Genbank) | URP |
| Escherichia coli HVH 50 (4-2593475) Taxon ID: 1280998 | 554706943 | ESK12388.1 (Genbank) | URP |
| Escherichia coli UMEA 3336-1 Taxon ID: 1281243 | 554701537 | ESK07057.1 (Genbank) | URP |
| Escherichia coli HVH 98 (4-5799287) Taxon ID: 1281034 | 554697360 | ESK02897.1 (Genbank) | URP |
| Escherichia coli JJ1886 Taxon ID: 1355100 | 554512906 | AGY85266.1 (Genbank) | URP |
| Escherichia coli A35218R Taxon ID: 1269009 | 553714746 | ESE29843.1 (Genbank) | URP |
| Escherichia coli 910096-2 Taxon ID: 1269010 | 553710592 | ESE25879.1 (Genbank) | URP |
| Escherichia coli 908675 Taxon ID: 1269004 | 553699824 | ESE15570.1 (Genbank) | URP |
| Escherichia coli 908691 Taxon ID: 1269005 | 553698293 | ESE14111.1 (Genbank) | URP |
| Escherichia coli 908658 Taxon ID: 1269003 | 553687238 | ESE03574.1 (Genbank) | URP |
| Escherichia coli 908632 Taxon ID: 1269002 | 553682991 | ESD99552.1 (Genbank) | URP |
| Escherichia coli 908624 Taxon ID: 1269001 | 553678511 | ESD95327.1 (Genbank) | URP |
| Escherichia coli 908573 Taxon ID: 1268998 | 553660882 | ESD78693.1 (Genbank) | URP |
| Escherichia coli 908541 Taxon ID: 1268996 | 553655577 | ESD73738.1 (Genbank) | URP |
| Escherichia coli 908555 Taxon ID: 1268997 | 553650785 | ESD69157.1 (Genbank) | URP |
| Escherichia coli 908525 Taxon ID: 1268995 | 553649283 | ESD67739.1 (Genbank) | URP |
| Escherichia coli 908524 Taxon ID: 1268994 | 553637351 | ESD56317.1 (Genbank) | URP |
| Escherichia coli 907889 Taxon ID: 1268989 | 553629850 | ESD49138.1 (Genbank) | URP |
| Escherichia coli 908519 Taxon ID: 1268991 | 553625467 | ESD44967.1 (Genbank) | URP |
| Escherichia coli 907710 Taxon ID: 1268985 | 553608532 | ESD28864.1 (Genbank) | URP |
| Escherichia coli 907701 Taxon ID: 1268984 | 553594907 | ESD15863.1 (Genbank) | URP |
| Escherichia coli 907700 Taxon ID: 1268983 | 553591544 | ESD12618.1 (Genbank) | URP |
| Escherichia coli 907672 Taxon ID: 1268982 | 553588086 | ESD09294.1 (Genbank) | URP |
| Escherichia coli 113302 Taxon ID: 1268977 | 553579718 | ESD01228.1 (Genbank) | URP |
| Escherichia coli 907446 Taxon ID: 1268981 | 553575355 | ESC96991.1 (Genbank) | URP |
| Escherichia coli 907779 Taxon ID: 1268988 | 553363911 | ESA90517.1 (Genbank) | URP |
| Escherichia coli 909945-2 Taxon ID: 1269007 | 553363031 | ESA89666.1 (Genbank) | URP |
| Escherichia coli 907357 Taxon ID: 1268979 | 553346955 | ESA74251.1 (Genbank) | URP |
| Escherichia coli 113290 Taxon ID: 1268976 | 553344768 | ESA72132.1 (Genbank) | URP |
| Escherichia coli 110957 Taxon ID: 1268975 | 553342935 | ESA70399.1 (Genbank) | URP |
| Escherichia coli 113303 Taxon ID: 1268978 | 553334764 | ESA62599.1 (Genbank) | URP |
| Escherichia coli SCD2 Taxon ID: 1337385 | 553019506 | ESA26411.1 (Genbank) | URP |
| Escherichia coli BIDMC 19C Taxon ID: 1328445 | 550207029 | ERO97491.1 (Genbank) | URP |
| Escherichia coli BWH 24 Taxon ID: 1328435 | 550201001 | ERO91586.1 (Genbank) | URP |
| Escherichia coli C321.deltaA Taxon ID: 1385755 | 549813936 | AGX34554.1 (Genbank) | URP |
| Escherichia coli LY180 Taxon ID: 1335916 | 544203006 | AGW09698.1 (Genbank) | URP |
| Escherichia coli O104:H21 str. CFSAN002237 Taxon ID: 1335304 | 540152122 | ERF97309.1 (Genbank) | URP |
| Escherichia coli O104:H21 str. CFSAN002236 Taxon ID: 1335303 | 540150393 | ERF95660.1 (Genbank) | URP |
| Escherichia coli TW07509 Taxon ID: 1105330 | 536874336 | ERC56539.1 (Genbank) | URP |
| Escherichia coli UMEA 3298-1 Taxon ID: 1281236 | 536036262 | ERB35060.1 (Genbank) | URP |
| Escherichia coli UMEA 3292-1 Taxon ID: 1281235 | 536035454 | ERB34274.1 (Genbank) | URP |
| Escherichia coli UMEA 3271-1 Taxon ID: 1281233 | 536028158 | ERB27133.1 (Genbank) | URP |
| Escherichia coli UMEA 3150-1 Taxon ID: 1281193 | 536021453 | ERB20581.1 (Genbank) | URP |
| Escherichia coli UMEA 3151-1 Taxon ID: 1281194 | 536020948 | ERB20087.1 (Genbank) | URP |
| Escherichia coli UMEA 3144-1 Taxon ID: 1281191 | 536014959 | ERB14171.1 (Genbank) | URP |
| Escherichia coli KOEGE 7 (16a) Taxon ID: 1281154 | 536006779 | ERB06167.1 (Genbank) | URP |
| Escherichia coli KOEGE 10 (25a) Taxon ID: 1281155 | 536005188 | ERB04596.1 (Genbank) | URP |
| Escherichia coli HVH 228 (4-7787030) Taxon ID: 1281152 | 535994155 | ERA93803.1 (Genbank) | URP |
| Escherichia coli HVH 210 (4-3042480) Taxon ID: 1281137 | 535989362 | ERA89054.1 (Genbank) | URP |
| Escherichia coli UMEA 4076-1 Taxon ID: 1281278 | 535918169 | ERA48749.1 (Genbank) | URP |
| Escherichia coli UMEA 3899-1 Taxon ID: 1281275 | 535911545 | ERA42321.1 (Genbank) | URP |
| Escherichia coli UMEA 4075-1 Taxon ID: 1281277 | 535904364 | ERA35317.1 (Genbank) | URP |
| Escherichia coli UMEA 3955-1 Taxon ID: 1281276 | 535901093 | ERA32133.1 (Genbank) | URP |
| Escherichia coli UMEA 3893-1 Taxon ID: 1281274 | 535889522 | ERA20824.1 (Genbank) | URP |
| Escherichia coli UMEA 3834-1 Taxon ID: 1281272 | 535888697 | ERA20007.1 (Genbank) | URP |
| Escherichia coli UMEA 3889-1 Taxon ID: 1281273 | 535886762 | ERA18094.1 (Genbank) | URP |
| Escherichia coli UMEA 3718-1 Taxon ID: 1281269 | 535868059 | EQZ99776.1 (Genbank) | URP |
| Escherichia coli UMEA 3703-1 Taxon ID: 1281266 | 535858127 | EQZ90077.1 (Genbank) | URP |
| Escherichia coli UMEA 3702-1 Taxon ID: 1281265 | 535846104 | EQZ78229.1 (Genbank) | URP |
| Escherichia coli UMEA 3671-1 Taxon ID: 1281260 | 535833105 | EQZ65423.1 (Genbank) | URP |
| Escherichia coli UMEA 3682-1 Taxon ID: 1281261 | 535831789 | EQZ64128.1 (Genbank) | URP |
| Escherichia coli UMEA 3656-1 Taxon ID: 1281258 | 535826302 | EQZ58749.1 (Genbank) | URP |
| Escherichia coli UMEA 3662-1 Taxon ID: 1281259 | 535821610 | EQZ54091.1 (Genbank) | URP |
| Escherichia coli UMEA 3632-1 Taxon ID: 1281256 | 535818197 | EQZ50711.1 (Genbank) | URP |
| Escherichia coli UMEA 3617-1 Taxon ID: 1281255 | 535806770 | EQZ39503.1 (Genbank) | URP |
| Escherichia coli UMEA 3609-1 Taxon ID: 1281254 | 535806129 | EQZ38870.1 (Genbank) | URP |
| Escherichia coli UMEA 3592-1 Taxon ID: 1281253 | 535802018 | EQZ34829.1 (Genbank) | URP |
| Escherichia coli UMEA 3585-1 Taxon ID: 1281252 | 535795143 | EQZ28100.1 (Genbank) | URP |
| Escherichia coli UMEA 3391-1 Taxon ID: 1281248 | 535785635 | EQZ18838.1 (Genbank) | URP |
| Escherichia coli UMEA 3355-1 Taxon ID: 1281247 | 535780253 | EQZ13514.1 (Genbank) | URP |
| Escherichia coli UMEA 3341-1 Taxon ID: 1281245 | 535777674 | EQZ10955.1 (Genbank) | URP |
| Escherichia coli UMEA 3318-1 Taxon ID: 1281240 | 535767305 | EQZ00834.1 (Genbank) | URP |
| Escherichia coli UMEA 3329-1 Taxon ID: 1281242 | 535764922 | EQY98477.1 (Genbank) | URP |
| Escherichia coli UMEA 3304-1 Taxon ID: 1281237 | 535756831 | EQY90616.1 (Genbank) | URP |
| Escherichia coli UMEA 3317-1 Taxon ID: 1281239 | 535754085 | EQY87912.1 (Genbank) | URP |
| Escherichia coli UMEA 3314-1 Taxon ID: 1281238 | 535753786 | EQY87621.1 (Genbank) | URP |
| Escherichia coli UMEA 3240-1 Taxon ID: 1281227 | 535723717 | EQY58340.1 (Genbank) | URP |
| Escherichia coli UMEA 3244-1 Taxon ID: 1281228 | 535720395 | EQY55048.1 (Genbank) | URP |
| Escherichia coli UMEA 3230-1 Taxon ID: 1281225 | 535707633 | EQY42558.1 (Genbank) | URP |
| Escherichia coli UMEA 3221-1 Taxon ID: 1281223 | 535704072 | EQY39059.1 (Genbank) | URP |
| Escherichia coli UMEA 3217-1 Taxon ID: 1281221 | 535698221 | EQY33362.1 (Genbank) | URP |
| Escherichia coli UMEA 3212-1 Taxon ID: 1281218 | 535682946 | EQY18347.1 (Genbank) | URP |
| Escherichia coli UMEA 3215-1 Taxon ID: 1281219 | 535679326 | EQY14814.1 (Genbank) | URP |
| Escherichia coli UMEA 3206-1 Taxon ID: 1281216 | 535665643 | EQY01461.1 (Genbank) | URP |
| Escherichia coli UMEA 3203-1 Taxon ID: 1281215 | 535663411 | EQX99261.1 (Genbank) | URP |
| Escherichia coli UMEA 3201-1 Taxon ID: 1281214 | 535657929 | EQX93872.1 (Genbank) | URP |
| Escherichia coli UMEA 3190-1 Taxon ID: 1281210 | 535655789 | EQX91811.1 (Genbank) | URP |
| Escherichia coli UMEA 3200-1 Taxon ID: 1281213 | 535650804 | EQX86934.1 (Genbank) | URP |
| Escherichia coli UMEA 3199-1 Taxon ID: 1281212 | 535646487 | EQX82680.1 (Genbank) | URP |
| Escherichia coli UMEA 3193-1 Taxon ID: 1281211 | 535639570 | EQX75926.1 (Genbank) | URP |
| Escherichia coli UMEA 3180-1 Taxon ID: 1281208 | 535637543 | EQX73939.1 (Genbank) | URP |
| Escherichia coli UMEA 3185-1 Taxon ID: 1281209 | 535628601 | EQX65176.1 (Genbank) | URP |
| Escherichia coli UMEA 3176-1 Taxon ID: 1281206 | 535619899 | EQX56652.1 (Genbank) | URP |
| Escherichia coli UMEA 3174-1 Taxon ID: 1281204 | 535613804 | EQX50637.1 (Genbank) | URP |
| Escherichia coli UMEA 3163-1 Taxon ID: 1281201 | 535594718 | EQX31952.1 (Genbank) | URP |
| Escherichia coli UMEA 3162-1 Taxon ID: 1281200 | 535589049 | EQX26366.1 (Genbank) | URP |
| Escherichia coli UMEA 3160-1 Taxon ID: 1281198 | 535582296 | EQX19706.1 (Genbank) | URP |
| Escherichia coli UMEA 3140-1 Taxon ID: 1281190 | 535572802 | EQX10360.1 (Genbank) | URP |
| Escherichia coli UMEA 3155-1 Taxon ID: 1281196 | 535566610 | EQX04283.1 (Genbank) | URP |
| Escherichia coli UMEA 3152-1 Taxon ID: 1281195 | 535563074 | EQX00798.1 (Genbank) | URP |
| Escherichia coli UMEA 3139-1 Taxon ID: 1281189 | 535554340 | EQW92287.1 (Genbank) | URP |
| Escherichia coli UMEA 3122-1 Taxon ID: 1281187 | 535546533 | EQW84647.1 (Genbank) | URP |
| Escherichia coli UMEA 3117-1 Taxon ID: 1281185 | 535539597 | EQW77816.1 (Genbank) | URP |
| Escherichia coli UMEA 3113-1 Taxon ID: 1281184 | 535530550 | EQW68904.1 (Genbank) | URP |
| Escherichia coli UMEA 3108-1 Taxon ID: 1281183 | 535526935 | EQW65332.1 (Genbank) | URP |
| Escherichia coli UMEA 3088-1 Taxon ID: 1281181 | 535521730 | EQW60196.1 (Genbank) | URP |
| Escherichia coli UMEA 3097-1 Taxon ID: 1281182 | 535516472 | EQW55056.1 (Genbank) | URP |
| Escherichia coli UMEA 3065-1 Taxon ID: 1281179 | 535504321 | EQW43419.1 (Genbank) | URP |
| Escherichia coli UMEA 3053-1 Taxon ID: 1281178 | 535501872 | EQW41139.1 (Genbank) | URP |
| Escherichia coli UMEA 3052-1 Taxon ID: 1281177 | 535492572 | EQW31956.1 (Genbank) | URP |
| Escherichia coli UMEA 3041-1 Taxon ID: 1281176 | 535491893 | EQW31280.1 (Genbank) | URP |
| Escherichia coli UMEA 3033-1 Taxon ID: 1281175 | 535486223 | EQW25636.1 (Genbank) | URP |
| Escherichia coli UMEA 3022-1 Taxon ID: 1281174 | 535478886 | EQW18426.1 (Genbank) | URP |
| Escherichia coli KOEGE 131 (358a) Taxon ID: 1281172 | 535471094 | EQW11133.1 (Genbank) | URP |
| Escherichia coli KOEGE 118 (317a) Taxon ID: 1281171 | 535466657 | EQW06747.1 (Genbank) | URP |
| Escherichia coli KOEGE 77 (202a) Taxon ID: 1281170 | 535462707 | EQW02801.1 (Genbank) | URP |
| Escherichia coli KOEGE 70 (185a) Taxon ID: 1281167 | 535443629 | EQV83999.1 (Genbank) | URP |
| Escherichia coli KOEGE 58 (171a) Taxon ID: 1281163 | 535428363 | EQV69068.1 (Genbank) | URP |
| Escherichia coli KOEGE 56 (169a) Taxon ID: 1281162 | 535425573 | EQV66317.1 (Genbank) | URP |
| Escherichia coli KOEGE 40 (102a) Taxon ID: 1281159 | 535409570 | EQV50851.1 (Genbank) | URP |
| Escherichia coli KOEGE 33 (68a) Taxon ID: 1281158 | 535404388 | EQV45781.1 (Genbank) | URP |
| Escherichia coli KOEGE 32 (66a) Taxon ID: 1281157 | 535400898 | EQV42345.1 (Genbank) | URP |
| Escherichia coli KOEGE 30 (63a) Taxon ID: 1281156 | 535391308 | EQV33008.1 (Genbank) | URP |
| Escherichia coli HVH 225 (4-1273116) Taxon ID: 1281150 | 535385505 | EQV27319.1 (Genbank) | URP |
| Escherichia coli HVH 222 (4-2977443) Taxon ID: 1281148 | 535370720 | EQV12834.1 (Genbank) | URP |
| Escherichia coli HVH 221 (4-3136817) Taxon ID: 1281147 | 535365355 | EQV07538.1 (Genbank) | URP |
| Escherichia coli HVH 217 (4-1022806) Taxon ID: 1281144 | 535350512 | EQU92939.1 (Genbank) | URP |
| Escherichia coli HVH 213 (4-3042928) Taxon ID: 1281140 | 535335770 | EQU78384.1 (Genbank) | URP |
| Escherichia coli HVH 209 (4-3062651) Taxon ID: 1281136 | 535329178 | EQU71906.1 (Genbank) | URP |
| Escherichia coli HVH 211 (4-3041891) Taxon ID: 1281138 | 535326550 | EQU69289.1 (Genbank) | URP |
| Escherichia coli HVH 205 (4-3094677) Taxon ID: 1281132 | 535304388 | EQU47285.1 (Genbank) | URP |
| Escherichia coli HVH 202 (4-3163997) Taxon ID: 1281129 | 535291583 | EQU34616.1 (Genbank) | URP |
| Escherichia coli HVH 203 (4-3126218) Taxon ID: 1281130 | 535290960 | EQU33995.1 (Genbank) | URP |
| Escherichia coli HVH 201 (4-4459431) Taxon ID: 1281128 | 535280123 | EQU23272.1 (Genbank) | URP |
| Escherichia coli HVH 200 (4-4449924) Taxon ID: 1281127 | 535279468 | EQU22625.1 (Genbank) | URP |
| Escherichia coli HVH 197 (4-4466217) Taxon ID: 1281124 | 535273532 | EQU16795.1 (Genbank) | URP |
| Escherichia coli HVH 198 (4-3206106) Taxon ID: 1281125 | 535270186 | EQU13486.1 (Genbank) | URP |
| Escherichia coli HVH 199 (4-5670322) Taxon ID: 1281126 | 535268047 | EQU11363.1 (Genbank) | URP |
| Escherichia coli HVH 196 (4-4530470) Taxon ID: 1281123 | 535261166 | EQU04587.1 (Genbank) | URP |
| Escherichia coli HVH 194 (4-2356805) Taxon ID: 1281121 | 535250612 | EQT94178.1 (Genbank) | URP |
| Escherichia coli HVH 195 (3-7155360) Taxon ID: 1281122 | 535250425 | EQT93994.1 (Genbank) | URP |
| Escherichia coli HVH 192 (4-3054470) Taxon ID: 1281119 | 535238550 | EQT82344.1 (Genbank) | URP |
| Escherichia coli HVH 191 (3-9341900) Taxon ID: 1281118 | 535234051 | EQT77770.1 (Genbank) | URP |
| Escherichia coli HVH 190 (4-3255514) Taxon ID: 1281117 | 535227550 | EQT71179.1 (Genbank) | URP |
| Escherichia coli HVH 187 (4-4471660) Taxon ID: 1281114 | 535221818 | EQT65578.1 (Genbank) | URP |
| Escherichia coli HVH 188 (4-2356988) Taxon ID: 1281115 | 535217151 | EQT61015.1 (Genbank) | URP |
| Escherichia coli HVH 186 (4-3405044) Taxon ID: 1281113 | 535211462 | EQT55416.1 (Genbank) | URP |
| Escherichia coli HVH 185 (4-2876639) Taxon ID: 1281112 | 535205388 | EQT49463.1 (Genbank) | URP |
| Escherichia coli HVH 184 (4-3343286) Taxon ID: 1281111 | 535200490 | EQT44670.1 (Genbank) | URP |
| Escherichia coli HVH 182 (4-0985554) Taxon ID: 1281109 | 535194031 | EQT38300.1 (Genbank) | URP |
| Escherichia coli HVH 180 (4-3051617) Taxon ID: 1281108 | 535183687 | EQT28122.1 (Genbank) | URP |
| Escherichia coli HVH 175 (4-3405184) Taxon ID: 1281104 | 535179362 | EQT23830.1 (Genbank) | URP |
| Escherichia coli HVH 176 (4-3428664) Taxon ID: 1281105 | 535176523 | EQT21017.1 (Genbank) | URP |
| Escherichia coli HVH 170 (4-3026949) Taxon ID: 1281100 | 535168749 | EQT13391.1 (Genbank) | URP |
| Escherichia coli HVH 171 (4-3191958) Taxon ID: 1281101 | 535155987 | EQT01061.1 (Genbank) | URP |
| Escherichia coli HVH 164 (4-5953081) Taxon ID: 1281097 | 535146466 | EQS91391.1 (Genbank) | URP |
| Escherichia coli HVH 162 (4-5627982) Taxon ID: 1281095 | 535133654 | EQS78961.1 (Genbank) | URP |
| Escherichia coli HVH 158 (4-3224287) Taxon ID: 1281091 | 535120142 | EQS66067.1 (Genbank) | URP |
| Escherichia coli HVH 154 (4-5636698) Taxon ID: 1281087 | 535119679 | EQS65610.1 (Genbank) | URP |
| Escherichia coli HVH 150 (4-3258106) Taxon ID: 1281083 | 535108443 | EQS54502.1 (Genbank) | URP |
| Escherichia coli HVH 153 (3-9344314) Taxon ID: 1281086 | 535104205 | EQS50237.1 (Genbank) | URP |
| Escherichia coli HVH 146 (4-3189767) Taxon ID: 1281079 | 535088460 | EQS34848.1 (Genbank) | URP |
| Escherichia coli HVH 147 (4-5893887) Taxon ID: 1281080 | 535086573 | EQS33012.1 (Genbank) | URP |
| Escherichia coli HVH 143 (4-5674999) Taxon ID: 1281076 | 535065179 | EQS11872.1 (Genbank) | URP |
| Escherichia coli HVH 141 (4-5995973) Taxon ID: 1281074 | 535058586 | EQS05416.1 (Genbank) | URP |
| Escherichia coli HVH 139 (4-3192644) Taxon ID: 1281072 | 535049400 | EQR96357.1 (Genbank) | URP |
| Escherichia coli HVH 138 (4-6066704) Taxon ID: 1281071 | 535046340 | EQR93339.1 (Genbank) | URP |
| Escherichia coli HVH 137 (4-2124971) Taxon ID: 1281070 | 535040333 | EQR87435.1 (Genbank) | URP |
| Escherichia coli HVH 128 (4-7030436) Taxon ID: 1281063 | 535011027 | EQR58592.1 (Genbank) | URP |
| Escherichia coli HVH 127 (4-7303629) Taxon ID: 1281062 | 535004536 | EQR52446.1 (Genbank) | URP |
| Escherichia coli HVH 126 (4-6034225) Taxon ID: 1281061 | 534999107 | EQR47172.1 (Genbank) | URP |
| Escherichia coli HVH 125 (4-2634716) Taxon ID: 1281060 | 534993590 | EQR41766.1 (Genbank) | URP |
| Escherichia coli HVH 121 (4-6877826) Taxon ID: 1281058 | 534989627 | EQR37869.1 (Genbank) | URP |
| Escherichia coli HVH 118 (4-7345399) Taxon ID: 1281055 | 534971462 | EQR19944.1 (Genbank) | URP |
| Escherichia coli HVH 115 (4-4465997) Taxon ID: 1281051 | 534952749 | EQR01523.1 (Genbank) | URP |
| Escherichia coli HVH 115 (4-4465989) Taxon ID: 1281052 | 534949270 | EQQ98142.1 (Genbank) | URP |
| Escherichia coli HVH 112 (4-5987253) Taxon ID: 1281048 | 534938456 | EQQ87464.1 (Genbank) | URP |
| Escherichia coli HVH 111 (4-7039018) Taxon ID: 1281047 | 534925693 | EQQ74981.1 (Genbank) | URP |
| Escherichia coli HVH 107 (4-5860571) Taxon ID: 1281043 | 534923880 | EQQ73189.1 (Genbank) | URP |
| Escherichia coli HVH 104 (4-6977960) Taxon ID: 1281040 | 534899348 | EQQ49079.1 (Genbank) | URP |
| Escherichia coli HVH 102 (4-6906788) Taxon ID: 1281038 | 534889358 | EQQ39341.1 (Genbank) | URP |
| Escherichia coli HVH 100 (4-2850729) Taxon ID: 1281036 | 534882227 | EQQ32368.1 (Genbank) | URP |
| Escherichia coli HVH 91 (4-4638751) Taxon ID: 1281028 | 534869332 | EQQ19750.1 (Genbank) | URP |
| Escherichia coli HVH 90 (4-3191362) Taxon ID: 1281027 | 534862934 | EQQ13491.1 (Genbank) | URP |
| Escherichia coli HVH 87 (4-5977630) Taxon ID: 1281024 | 534856230 | EQQ06875.1 (Genbank) | URP |
| Escherichia coli HVH 84 (4-1021478) Taxon ID: 1281021 | 534842341 | EQP93297.1 (Genbank) | URP |
| Escherichia coli HVH 82 (4-2209276) Taxon ID: 1281019 | 534836601 | EQP87605.1 (Genbank) | URP |
| Escherichia coli HVH 80 (4-2428830) Taxon ID: 1281018 | 534832783 | EQP83868.1 (Genbank) | URP |
| Escherichia coli HVH 79 (4-2512823) Taxon ID: 1281017 | 534820202 | EQP71669.1 (Genbank) | URP |
| Escherichia coli HVH 78 (4-2735946) Taxon ID: 1281016 | 534817727 | EQP69230.1 (Genbank) | URP |
| Escherichia coli HVH 76 (4-2538717) Taxon ID: 1281014 | 534809023 | EQP60866.1 (Genbank) | URP |
| Escherichia coli HVH 73 (4-2393174) Taxon ID: 1281012 | 534798938 | EQP51179.1 (Genbank) | URP |
| Escherichia coli HVH 70 (4-2963531) Taxon ID: 1281011 | 534797601 | EQP49873.1 (Genbank) | URP |
| Escherichia coli HVH 65 (4-2262045) Taxon ID: 1281008 | 534783041 | EQP35549.1 (Genbank) | URP |
| Escherichia coli HVH 59 (4-1119338) Taxon ID: 1281005 | 534764717 | EQP17458.1 (Genbank) | URP |
| Escherichia coli HVH 56 (4-2153033) Taxon ID: 1281003 | 534754949 | EQP07801.1 (Genbank) | URP |
| Escherichia coli HVH 53 (4-0631051) Taxon ID: 1281000 | 534748572 | EQP01540.1 (Genbank) | URP |
| Escherichia coli HVH 55 (4-2646161) Taxon ID: 1281002 | 534744919 | EQO97940.1 (Genbank) | URP |
| Escherichia coli HVH 46 (4-2758776) Taxon ID: 1280996 | 534733269 | EQO86492.1 (Genbank) | URP |
| Escherichia coli HVH 48 (4-2658593) Taxon ID: 1280997 | 534730526 | EQO83786.1 (Genbank) | URP |
| Escherichia coli HVH 44 (4-2298570) Taxon ID: 1280994 | 534717301 | EQO70797.1 (Genbank) | URP |
| Escherichia coli HVH 43 (4-2173468) Taxon ID: 1280993 | 534714231 | EQO67771.1 (Genbank) | URP |
| Escherichia coli HVH 41 (4-2677849) Taxon ID: 1280991 | 534709515 | EQO63162.1 (Genbank) | URP |
| Escherichia coli HVH 42 (4-2100061) Taxon ID: 1280992 | 534706431 | EQO60118.1 (Genbank) | URP |
| Escherichia coli HVH 38 (4-2774682) Taxon ID: 1280988 | 534690426 | EQO46622.1 (Genbank) | URP |
| Escherichia coli HVH 35 (4-2962667) Taxon ID: 1280985 | 534674933 | EQO30949.1 (Genbank) | URP |
| Escherichia coli HVH 33 (4-2174936) Taxon ID: 1280984 | 534672922 | EQO28951.1 (Genbank) | URP |
| Escherichia coli HVH 32 (4-3773988) Taxon ID: 1280983 | 534665453 | EQO21604.1 (Genbank) | URP |
| Escherichia coli HVH 30 (4-2661829) Taxon ID: 1280981 | 534659481 | EQO15731.1 (Genbank) | URP |
| Escherichia coli HVH 28 (4-0907367) Taxon ID: 1280979 | 534657736 | EQO14002.1 (Genbank) | URP |
| Escherichia coli HVH 25 (4-5851939) Taxon ID: 1280976 | 534635880 | EQN92459.1 (Genbank) | URP |
| Escherichia coli HVH 26 (4-5703913) Taxon ID: 1280977 | 534635703 | EQN92285.1 (Genbank) | URP |
| Escherichia coli HVH 24 (4-5985145) Taxon ID: 1280975 | 534627428 | EQN84180.1 (Genbank) | URP |
| Escherichia coli HVH 21 (4-4517873) Taxon ID: 1280972 | 534617500 | EQN74341.1 (Genbank) | URP |
| Escherichia coli HVH 19 (4-7154984) Taxon ID: 1280970 | 534611522 | EQN68510.1 (Genbank) | URP |
| Escherichia coli HVH 18 (4-8589585) Taxon ID: 1280969 | 534608172 | EQN65207.1 (Genbank) | URP |
| Escherichia coli HVH 17 (4-7473087) Taxon ID: 1280968 | 534594784 | EQN52688.1 (Genbank) | URP |
| Escherichia coli HVH 9 (4-6942539) Taxon ID: 1280963 | 534572055 | EQN31028.1 (Genbank) | URP |
| Escherichia coli HVH 6 (3-8296502) Taxon ID: 1280961 | 534566293 | EQN25549.1 (Genbank) | URP |
| Escherichia coli HVH 4 (4-7276109) Taxon ID: 1280959 | 534558603 | EQN18172.1 (Genbank) | URP |
| Escherichia coli HVH 5 (4-7148410) Taxon ID: 1280960 | 534557762 | EQN17340.1 (Genbank) | URP |
| Escherichia coli HVH 1 (4-6876161) Taxon ID: 1280956 | 534552056 | EQN11657.1 (Genbank) | URP |
| Escherichia coli HVH 3 (4-7276001) Taxon ID: 1280958 | 534547430 | EQN07053.1 (Genbank) | URP |
| Escherichia coli E2265 Taxon ID: 1331062 | 514338694 | EPH50844.1 (Genbank) | URP |
| Escherichia coli KTE186 Taxon ID: 1181732 | 508561774 | EOX29007.1 (Genbank) | URP |
| Escherichia coli KTE185 Taxon ID: 1181731 | 508555332 | EOX22614.1 (Genbank) | URP |
| Escherichia coli KTE240 Taxon ID: 1181782 | 508543713 | EOX12032.1 (Genbank) | URP |
| Escherichia coli KTE182 Taxon ID: 1181728 | 508528023 | EOW96572.1 (Genbank) | URP |
| Escherichia coli KTE155 Taxon ID: 1182729 | 508489391 | EOW58586.1 (Genbank) | URP |
| Escherichia coli KTE132 Taxon ID: 1182716 | 508476776 | EOW46167.1 (Genbank) | URP |
| Escherichia coli KTE130 Taxon ID: 1182715 | 508474988 | EOW44411.1 (Genbank) | URP |
| Escherichia coli KTE126 Taxon ID: 1182713 | 508464863 | EOW34453.1 (Genbank) | URP |
| Escherichia coli KTE121 Taxon ID: 1182711 | 508460899 | EOW30553.1 (Genbank) | URP |
| Escherichia coli KTE107 Taxon ID: 1182703 | 508451654 | EOW21461.1 (Genbank) | URP |
| Escherichia coli KTE100 Taxon ID: 1182699 | 508435856 | EOW05951.1 (Genbank) | URP |
| Escherichia coli KTE102 Taxon ID: 1182701 | 508433843 | EOW03965.1 (Genbank) | URP |
| Escherichia coli KTE98 Taxon ID: 1182698 | 508429970 | EOW00072.1 (Genbank) | URP |
| Escherichia coli KTE89 Taxon ID: 1182693 | 508419810 | EOV90122.1 (Genbank) | URP |
| Escherichia coli KTE74 Taxon ID: 1182681 | 508418893 | EOV89219.1 (Genbank) | URP |
| Escherichia coli KTE73 Taxon ID: 1182680 | 508406823 | EOV77407.1 (Genbank) | URP |
| Escherichia coli KTE70 Taxon ID: 1182677 | 508401689 | EOV72375.1 (Genbank) | URP |
| Escherichia coli KTE69 Taxon ID: 1182676 | 508392892 | EOV63796.1 (Genbank) | URP |
| Escherichia coli KTE64 Taxon ID: 1182671 | 508385855 | EOV56887.1 (Genbank) | URP |
| Escherichia coli KTE61 Taxon ID: 1182668 | 508379673 | EOV50808.1 (Genbank) | URP |
| Escherichia coli KTE221 Taxon ID: 1181767 | 508366875 | EOV38214.1 (Genbank) | URP |
| Escherichia coli KTE219 Taxon ID: 1181765 | 508362595 | EOV33965.1 (Genbank) | URP |
| Escherichia coli KTE222 Taxon ID: 1181768 | 508362409 | EOV33781.1 (Genbank) | URP |
| Escherichia coli KTE199 Taxon ID: 1181745 | 508353980 | EOV25487.1 (Genbank) | URP |
| Escherichia coli KTE198 Taxon ID: 1181744 | 508346106 | EOV17715.1 (Genbank) | URP |
| Escherichia coli KTE40 Taxon ID: 1169357 | 508339816 | EOV11553.1 (Genbank) | URP |
| Escherichia coli KTE195 Taxon ID: 1181741 | 508335094 | EOV06902.1 (Genbank) | URP |
| Escherichia coli KTE38 Taxon ID: 1169355 | 508333581 | EOV05417.1 (Genbank) | URP |
| Escherichia coli KTE34 Taxon ID: 1169352 | 508319778 | EOU91885.1 (Genbank) | URP |
| Escherichia coli KTE37 Taxon ID: 1169354 | 508319652 | EOU91760.1 (Genbank) | URP |
| Escherichia coli KTE36 Taxon ID: 1169353 | 508317877 | EOU90008.1 (Genbank) | URP |
| Escherichia coli KTE27 Taxon ID: 1169346 | 508303857 | EOU76310.1 (Genbank) | URP |
| Escherichia coli KTE24 Taxon ID: 1169343 | 508299264 | EOU71769.1 (Genbank) | URP |
| Escherichia coli KTE20 Taxon ID: 1169339 | 508293365 | EOU65967.1 (Genbank) | URP |
| Escherichia coli KTE19 Taxon ID: 1169338 | 508289202 | EOU62129.1 (Genbank) | URP |
| Escherichia coli KTE14 Taxon ID: 1169333 | 508284607 | EOU57605.1 (Genbank) | URP |
| Escherichia coli KTE35 Taxon ID: 1169320 | 508274343 | EOU47486.1 (Genbank) | URP |
| Escherichia coli KTE3 Taxon ID: 1169316 | 508261410 | EOU34739.1 (Genbank) | URP |
| Escherichia coli KTE13 Taxon ID: 1169318 | 508258653 | EOU32009.1 (Genbank) | URP |
| Escherichia coli KTE7 Taxon ID: 1169317 | 508258466 | EOU31825.1 (Genbank) | URP |
| Escherichia coli O157:H43 str. T22 Taxon ID: 1137135 | 478699040 | ENO11194.1 (Genbank) | URP |
| Escherichia coli p0305293.6 Taxon ID: 1116094 | 477495038 | ENH56039.1 (Genbank) | URP |
| Escherichia coli p0305293.7 Taxon ID: 1116095 | 477489848 | ENH51173.1 (Genbank) | URP |
| Escherichia coli p0305293.5 Taxon ID: 1116093 | 477483116 | ENH45006.1 (Genbank) | URP |
| Escherichia coli P0304816.5 Taxon ID: 1116181 | 477477230 | ENH39409.1 (Genbank) | URP |
| Escherichia coli P0304816.3 Taxon ID: 1116179 | 477469629 | ENH32038.1 (Genbank) | URP |
| Escherichia coli P0304816.4 Taxon ID: 1116180 | 477469473 | ENH31884.1 (Genbank) | URP |
| Escherichia coli P0302308.12 Taxon ID: 1116160 | 477455456 | ENH18586.1 (Genbank) | URP |
| Escherichia coli P0302308.13 Taxon ID: 1116161 | 477453681 | ENH16849.1 (Genbank) | URP |
| Escherichia coli P0301867.7 Taxon ID: 1116070 | 477444527 | ENH08315.1 (Genbank) | URP |
| Escherichia coli 178850 Taxon ID: 1116106 | 477427497 | ENG92157.1 (Genbank) | URP |
| Escherichia coli 178200 Taxon ID: 1116107 | 477416509 | ENG82024.1 (Genbank) | URP |
| Escherichia coli p0305293.9 Taxon ID: 1116097 | 477410798 | ENG76804.1 (Genbank) | URP |
| Escherichia coli p0305293.8 Taxon ID: 1116096 | 477404372 | ENG70902.1 (Genbank) | URP |
| Escherichia coli p0305293.4 Taxon ID: 1116092 | 477397647 | ENG64513.1 (Genbank) | URP |
| Escherichia coli p0305293.3 Taxon ID: 1116091 | 477394647 | ENG61663.1 (Genbank) | URP |
| Escherichia coli p0305293.2 Taxon ID: 1116090 | 477387740 | ENG55067.1 (Genbank) | URP |
| Escherichia coli p0305293.15 Taxon ID: 1116102 | 477383239 | ENG50780.1 (Genbank) | URP |
| Escherichia coli p0305293.12 Taxon ID: 1116100 | 477374363 | ENG42476.1 (Genbank) | URP |
| Escherichia coli p0305293.11 Taxon ID: 1116099 | 477373453 | ENG41587.1 (Genbank) | URP |
| Escherichia coli P0305260.9 Taxon ID: 1116083 | 477364915 | ENG33354.1 (Genbank) | URP |
| Escherichia coli p0305293.10 Taxon ID: 1116098 | 477361871 | ENG30408.1 (Genbank) | URP |
| Escherichia coli P0305260.6 Taxon ID: 1116080 | 477347460 | ENG16421.1 (Genbank) | URP |
| Escherichia coli P0305260.5 Taxon ID: 1116194 | 477342740 | ENG11817.1 (Genbank) | URP |
| Escherichia coli P0305260.4 Taxon ID: 1116193 | 477334261 | ENG03718.1 (Genbank) | URP |
| Escherichia coli P0305260.3 Taxon ID: 1116192 | 477333044 | ENG02547.1 (Genbank) | URP |
| Escherichia coli P0305260.13 Taxon ID: 1116087 | 477320238 | ENF90309.1 (Genbank) | URP |
| Escherichia coli P0305260.12 Taxon ID: 1116086 | 477314643 | ENF84918.1 (Genbank) | URP |
| Escherichia coli P0305260.11 Taxon ID: 1116085 | 477312195 | ENF82555.1 (Genbank) | URP |
| Escherichia coli P0305260.10 Taxon ID: 1116084 | 477304946 | ENF75742.1 (Genbank) | URP |
| Escherichia coli P0304816.9 Taxon ID: 1116185 | 477300089 | ENF70972.1 (Genbank) | URP |
| Escherichia coli P0304816.8 Taxon ID: 1116184 | 477296841 | ENF67830.1 (Genbank) | URP |
| Escherichia coli P0304816.7 Taxon ID: 1116183 | 477290226 | ENF61529.1 (Genbank) | URP |
| Escherichia coli P0304816.6 Taxon ID: 1116182 | 477279250 | ENF51230.1 (Genbank) | URP |
| Escherichia coli P0304816.14 Taxon ID: 1116190 | 477261067 | ENF33774.1 (Genbank) | URP |
| Escherichia coli P0304816.12 Taxon ID: 1116188 | 477257288 | ENF30128.1 (Genbank) | URP |
| Escherichia coli P0304816.10 Taxon ID: 1116186 | 477250372 | ENF23624.1 (Genbank) | URP |
| Escherichia coli P0304777.9 Taxon ID: 1116170 | 477237649 | ENF11611.1 (Genbank) | URP |
| Escherichia coli P0304777.8 Taxon ID: 1116169 | 477234615 | ENF08721.1 (Genbank) | URP |
| Escherichia coli P0304777.5 Taxon ID: 1116167 | 477226545 | ENF01233.1 (Genbank) | URP |
| Escherichia coli P0304777.7 Taxon ID: 1116168 | 477225074 | ENE99809.1 (Genbank) | URP |
| Escherichia coli P0304777.3 Taxon ID: 1116165 | 477210946 | ENE86557.1 (Genbank) | URP |
| Escherichia coli P0304777.2 Taxon ID: 1116164 | 477205079 | ENE80886.1 (Genbank) | URP |
| Escherichia coli P0304777.15 Taxon ID: 1116176 | 477201439 | ENE77376.1 (Genbank) | URP |
| Escherichia coli P0304777.14 Taxon ID: 1116175 | 477195005 | ENE71079.1 (Genbank) | URP |
| Escherichia coli P0304777.13 Taxon ID: 1116174 | 477189251 | ENE65440.1 (Genbank) | URP |
| Escherichia coli P0304777.12 Taxon ID: 1116173 | 477187810 | ENE64044.1 (Genbank) | URP |
| Escherichia coli P0304777.11 Taxon ID: 1116172 | 477179953 | ENE56563.1 (Genbank) | URP |
| Escherichia coli P0302293.9 Taxon ID: 1116078 | 477174297 | ENE51090.1 (Genbank) | URP |
| Escherichia coli P0302293.8 Taxon ID: 1116077 | 477163798 | ENE40934.1 (Genbank) | URP |
| Escherichia coli P0302293.6 Taxon ID: 1116076 | 477158051 | ENE35516.1 (Genbank) | URP |
| Escherichia coli P0302293.4 Taxon ID: 1116075 | 477151510 | ENE29308.1 (Genbank) | URP |
| Escherichia coli P0302293.3 Taxon ID: 1116074 | 477143843 | ENE22046.1 (Genbank) | URP |
| Escherichia coli p0305293.14 Taxon ID: 1125646 | 477130987 | ENE10065.1 (Genbank) | URP |
| Escherichia coli P0304799.3 Taxon ID: 1125651 | 477128768 | ENE07939.1 (Genbank) | URP |
| Escherichia coli P0305260.2 Taxon ID: 1125645 | 477128174 | ENE07358.1 (Genbank) | URP |
| Escherichia coli P0302293.7 Taxon ID: 1125643 | 477118893 | END98483.1 (Genbank) | URP |
| Escherichia coli P0301867.13 Taxon ID: 1125642 | 477110971 | END90998.1 (Genbank) | URP |
| Escherichia coli P0299483.3 Taxon ID: 1125650 | 477101596 | END82058.1 (Genbank) | URP |
| Escherichia coli P0299483.2 Taxon ID: 1125649 | 477098255 | END78825.1 (Genbank) | URP |
| Escherichia coli P0299483.1 Taxon ID: 1125648 | 477087009 | END67947.1 (Genbank) | URP |
| Escherichia coli P0298942.3 Taxon ID: 1125639 | 477086759 | END67698.1 (Genbank) | URP |
| Escherichia coli BCE006_MS-23 Taxon ID: 1125637 | 477075836 | END57423.1 (Genbank) | URP |
| Escherichia coli p0305293.13 Taxon ID: 1116101 | 477061012 | END43516.1 (Genbank) | URP |
| Escherichia coli 2733950 Taxon ID: 1125635 | 477059613 | END42190.1 (Genbank) | URP |
| Escherichia coli 2854350 Taxon ID: 1125636 | 477057676 | END40331.1 (Genbank) | URP |
| Escherichia coli P0302308.4 Taxon ID: 1116156 | 477041731 | END25661.1 (Genbank) | URP |
| Escherichia coli P0302308.5 Taxon ID: 1116157 | 477037569 | END21739.1 (Genbank) | URP |
| Escherichia coli P0302308.2 Taxon ID: 1116154 | 477029728 | END14484.1 (Genbank) | URP |
| Escherichia coli P0302308.3 Taxon ID: 1116155 | 477025412 | END10279.1 (Genbank) | URP |
| Escherichia coli P0302308.11 Taxon ID: 1116159 | 477016863 | END02155.1 (Genbank) | URP |
| Escherichia coli P0302308.10 Taxon ID: 1116158 | 477013020 | ENC98435.1 (Genbank) | URP |
| Escherichia coli P0301867.8 Taxon ID: 1116071 | 477009401 | ENC94951.1 (Genbank) | URP |
| Escherichia coli P0301867.11 Taxon ID: 1116072 | 477004859 | ENC90752.1 (Genbank) | URP |
| Escherichia coli P0299917.9 Taxon ID: 1116055 | 476996941 | ENC83282.1 (Genbank) | URP |
| Escherichia coli P0299917.7 Taxon ID: 1116053 | 476991733 | ENC78321.1 (Genbank) | URP |
| Escherichia coli P0299917.6 Taxon ID: 1116052 | 476983683 | ENC70534.1 (Genbank) | URP |
| Escherichia coli P0299917.8 Taxon ID: 1116054 | 476983512 | ENC70366.1 (Genbank) | URP |
| Escherichia coli P0299917.3 Taxon ID: 1116049 | 476966409 | ENC54116.1 (Genbank) | URP |
| Escherichia coli P0299917.2 Taxon ID: 1116048 | 476957864 | ENC46156.1 (Genbank) | URP |
| Escherichia coli P0299917.10 Taxon ID: 1116056 | 476950812 | ENC39640.1 (Genbank) | URP |
| Escherichia coli P0299438.9 Taxon ID: 1116044 | 476941942 | ENC31161.1 (Genbank) | URP |
| Escherichia coli P0299438.8 Taxon ID: 1116043 | 476935043 | ENC24465.1 (Genbank) | URP |
| Escherichia coli P0299438.7 Taxon ID: 1116042 | 476927909 | ENC17508.1 (Genbank) | URP |
| Escherichia coli P0299438.5 Taxon ID: 1116040 | 476920818 | ENC10564.1 (Genbank) | URP |
| Escherichia coli P0299438.3 Taxon ID: 1116038 | 476908892 | ENB99066.1 (Genbank) | URP |
| Escherichia coli P0298942.9 Taxon ID: 1116146 | 476887320 | ENB78748.1 (Genbank) | URP |
| Escherichia coli P0298942.7 Taxon ID: 1116144 | 476886342 | ENB77823.1 (Genbank) | URP |
| Escherichia coli P0298942.8 Taxon ID: 1116145 | 476884913 | ENB76475.1 (Genbank) | URP |
| Escherichia coli P0298942.2 Taxon ID: 1116142 | 476871603 | ENB64166.1 (Genbank) | URP |
| Escherichia coli P0298942.11 Taxon ID: 1116148 | 476852657 | ENB47772.1 (Genbank) | URP |
| Escherichia coli P0298942.10 Taxon ID: 1116147 | 476841323 | ENB40128.1 (Genbank) | URP |
| Escherichia coli MP021561.3 Taxon ID: 1116137 | 476838007 | ENB36924.1 (Genbank) | URP |
| Escherichia coli BCE032_MS-12 Taxon ID: 1116117 | 476834945 | ENB33954.1 (Genbank) | URP |
| Escherichia coli BCE030_MS-09 Taxon ID: 1116116 | 476827799 | ENB27174.1 (Genbank) | URP |
| Escherichia coli BCE011_MS-01 Taxon ID: 1116114 | 476821597 | ENB21398.1 (Genbank) | URP |
| Escherichia coli BCE008_MS-01 Taxon ID: 1116110 | 476814649 | ENB15175.1 (Genbank) | URP |
| Escherichia coli 2875150 Taxon ID: 1116036 | 476811856 | ENB12609.1 (Genbank) | URP |
| Escherichia coli 2866350 Taxon ID: 1116027 | 476805559 | ENB06621.1 (Genbank) | URP |
| Escherichia coli 2862600 Taxon ID: 1116024 | 476796265 | ENA97772.1 (Genbank) | URP |
| Escherichia coli 2864350 Taxon ID: 1116025 | 476793312 | ENA94894.1 (Genbank) | URP |
| Escherichia coli 2741950 Taxon ID: 1116009 | 476777550 | ENA79806.1 (Genbank) | URP |
| Escherichia coli 2730450 Taxon ID: 1116006 | 476776087 | ENA78391.1 (Genbank) | URP |
| Escherichia coli 180200 Taxon ID: 1116104 | 476764154 | ENA67470.1 (Genbank) | URP |
| Escherichia coli 179550 Taxon ID: 1116109 | 476760172 | ENA63713.1 (Genbank) | URP |
| Escherichia coli 178900 Taxon ID: 1116108 | 476757404 | ENA61090.1 (Genbank) | URP |
| Escherichia coli 2726950 Taxon ID: 1116123 | 476748050 | ENA52238.1 (Genbank) | URP |
| Escherichia coli 2729250 Taxon ID: 1116004 | 476747226 | ENA51430.1 (Genbank) | URP |
| Escherichia coli P0301867.2 Taxon ID: 1125641 | 476740026 | ENA44473.1 (Genbank) | URP |
| Escherichia coli P0301867.4 Taxon ID: 1116068 | 476734347 | ENA39009.1 (Genbank) | URP |
| Escherichia coli BCE007_MS-11 Taxon ID: 1116113 | 476726748 | ENA31537.1 (Genbank) | URP |
| Escherichia coli BCE008_MS-13 Taxon ID: 1116471 | 476710435 | ENA15468.1 (Genbank) | URP |
| Escherichia coli P0298942.1 Taxon ID: 1116141 | 476709843 | ENA14879.1 (Genbank) | URP |
| Escherichia coli P0299917.1 Taxon ID: 1116047 | 476701691 | ENA06848.1 (Genbank) | URP |
| Escherichia coli P0299438.2 Taxon ID: 1116152 | 476698255 | ENA03438.1 (Genbank) | URP |
| Escherichia coli P0304816.1 Taxon ID: 1116177 | 476691677 | EMZ96972.1 (Genbank) | URP |
| Escherichia coli P0305260.1 Taxon ID: 1125644 | 476686511 | EMZ91873.1 (Genbank) | URP |
| Escherichia coli p0305293.1 Taxon ID: 1116089 | 476679016 | EMZ84623.1 (Genbank) | URP |
| Escherichia coli 2722950 Taxon ID: 1116121 | 476674670 | EMZ80320.1 (Genbank) | URP |
| Escherichia coli 199900.1 Taxon ID: 1116130 | 476672378 | EMZ78084.1 (Genbank) | URP |
| Escherichia coli 2846750 Taxon ID: 1116126 | 476663270 | EMZ69256.1 (Genbank) | URP |
| Escherichia coli 174900 Taxon ID: 1116003 | 476657424 | EMZ63530.1 (Genbank) | URP |
| Escherichia coli SWW33 Taxon ID: 1235805 | 476637671 | EMZ44140.1 (Genbank) | URP |
| Escherichia coli 2720900 Taxon ID: 1116120 | 476376111 | EMX93225.1 (Genbank) | URP |
| Escherichia coli BCE001_MS16 Taxon ID: 1116037 | 476372961 | EMX90092.1 (Genbank) | URP |
| Escherichia coli 2719100 Taxon ID: 1116119 | 476369773 | EMX86912.1 (Genbank) | URP |
| Escherichia coli 2726800 Taxon ID: 1116122 | 476359842 | EMX77096.1 (Genbank) | URP |
| Escherichia coli Envira 8/11 Taxon ID: 1115997 | 476354039 | EMX71342.1 (Genbank) | URP |
| Escherichia coli Envira 10/1 Taxon ID: 1115998 | 476352398 | EMX69711.1 (Genbank) | URP |
| Escherichia coli Jurua 18/11 Taxon ID: 1115999 | 476345606 | EMX62974.1 (Genbank) | URP |
| Escherichia coli MP020940.1 Taxon ID: 1116140 | 476338569 | EMX56004.1 (Genbank) | URP |
| Escherichia coli Jurua 20/10 Taxon ID: 1116000 | 476334978 | EMX52435.1 (Genbank) | URP |
| Escherichia coli MP020980.2 Taxon ID: 1116139 | 476330886 | EMX48362.1 (Genbank) | URP |
| Escherichia coli MP021017.1 Taxon ID: 1116057 | 476320805 | EMX38395.1 (Genbank) | URP |
| Escherichia coli MP021552.8 Taxon ID: 1116133 | 476320328 | EMX37923.1 (Genbank) | URP |
| Escherichia coli MP021561.2 Taxon ID: 1116136 | 476313637 | EMX31330.1 (Genbank) | URP |
| Escherichia coli MP021566.1 Taxon ID: 1116138 | 476306653 | EMX24483.1 (Genbank) | URP |
| Escherichia coli P0301867.1 Taxon ID: 1116066 | 476302520 | EMX20400.1 (Genbank) | URP |
| Escherichia coli P0302293.2 Taxon ID: 1116073 | 476295933 | EMX13896.1 (Genbank) | URP |
| Escherichia coli P0302308.1 Taxon ID: 1116153 | 476289694 | EMX07728.1 (Genbank) | URP |
| Escherichia coli 174750 Taxon ID: 1125634 | 476283806 | EMX01921.1 (Genbank) | URP |
| Escherichia coli P0304777.1 Taxon ID: 1116163 | 476281446 | EMW99572.1 (Genbank) | URP |
| Escherichia coli ThroopD Taxon ID: 1116001 | 476278368 | EMW96521.1 (Genbank) | URP |
| Escherichia coli 180050 Taxon ID: 1116103 | 476268726 | EMW87190.1 (Genbank) | URP |
| Escherichia coli 180600 Taxon ID: 1116105 | 476261004 | EMW79647.1 (Genbank) | URP |
| Escherichia coli 2731150 Taxon ID: 1116007 | 476259746 | EMW78413.1 (Genbank) | URP |
| Escherichia coli 2747800 Taxon ID: 1116010 | 476255434 | EMW74171.1 (Genbank) | URP |
| Escherichia coli 2749250 Taxon ID: 1116011 | 476248526 | EMW67516.1 (Genbank) | URP |
| Escherichia coli 2756500 Taxon ID: 1116012 | 476241059 | EMW60329.1 (Genbank) | URP |
| Escherichia coli 2762100 Taxon ID: 1116013 | 476238066 | EMW57412.1 (Genbank) | URP |
| Escherichia coli 2770900 Taxon ID: 1116014 | 476232223 | EMW51685.1 (Genbank) | URP |
| Escherichia coli 2780750 Taxon ID: 1116015 | 476228777 | EMW48295.1 (Genbank) | URP |
| Escherichia coli 2788150 Taxon ID: 1116017 | 476221747 | EMW41571.1 (Genbank) | URP |
| Escherichia coli 2785200 Taxon ID: 1116016 | 476214734 | EMW34805.1 (Genbank) | URP |
| Escherichia coli 2848050 Taxon ID: 1116127 | 476202990 | EMW23396.1 (Genbank) | URP |
| Escherichia coli 2845650 Taxon ID: 1116125 | 476198331 | EMW18802.1 (Genbank) | URP |
| Escherichia coli 2850400 Taxon ID: 1116018 | 476197744 | EMW18223.1 (Genbank) | URP |
| Escherichia coli 2851500 Taxon ID: 1116128 | 476181393 | EMW02332.1 (Genbank) | URP |
| Escherichia coli 2853500 Taxon ID: 1116020 | 476180915 | EMW01862.1 (Genbank) | URP |
| Escherichia coli 2860050 Taxon ID: 1116021 | 476171667 | EMV92809.1 (Genbank) | URP |
| Escherichia coli 2865200 Taxon ID: 1116026 | 476168372 | EMV89578.1 (Genbank) | URP |
| Escherichia coli 2861200 Taxon ID: 1116023 | 476162794 | EMV84142.1 (Genbank) | URP |
| Escherichia coli 2866750 Taxon ID: 1116030 | 476153426 | EMV75160.1 (Genbank) | URP |
| Escherichia coli 2867750 Taxon ID: 1116031 | 476135482 | EMV57581.1 (Genbank) | URP |
| Escherichia coli 2871950 Taxon ID: 1116032 | 476135063 | EMV57167.1 (Genbank) | URP |
| Escherichia coli 2872000 Taxon ID: 1116033 | 476134840 | EMV56947.1 (Genbank) | URP |
| Escherichia coli 2872800 Taxon ID: 1116034 | 476123333 | EMV45936.1 (Genbank) | URP |
| Escherichia coli BCE019_MS-13 Taxon ID: 1116115 | 476117755 | EMV40476.1 (Genbank) | URP |
| Escherichia coli 2875000 Taxon ID: 1116035 | 476115806 | EMV38599.1 (Genbank) | URP |
| Escherichia coli BCE002_MS12 Taxon ID: 1116112 | 476108993 | EMV31900.1 (Genbank) | URP |
| Escherichia coli MP021017.12 Taxon ID: 1116111 | 476098437 | EMV21846.1 (Genbank) | URP |
| Escherichia coli BCE034_MS-14 Taxon ID: 1116118 | 476097940 | EMV21355.1 (Genbank) | URP |
| Escherichia coli MP021017.11 Taxon ID: 1116065 | 476086615 | EMV10405.1 (Genbank) | URP |
| Escherichia coli MP021017.10 Taxon ID: 1116064 | 476081530 | EMV05498.1 (Genbank) | URP |
| Escherichia coli MP021017.3 Taxon ID: 1116059 | 476069546 | EMU94015.1 (Genbank) | URP |
| Escherichia coli MP021017.4 Taxon ID: 1116060 | 476067689 | EMU92189.1 (Genbank) | URP |
| Escherichia coli MP021017.5 Taxon ID: 1116061 | 476057466 | EMU82193.1 (Genbank) | URP |
| Escherichia coli MP021017.9 Taxon ID: 1116063 | 476052472 | EMU77299.1 (Genbank) | URP |
| Escherichia coli MP021552.12 Taxon ID: 1116135 | 476043778 | EMU69022.1 (Genbank) | URP |
| Escherichia coli MP021552.7 Taxon ID: 1116132 | 476035243 | EMU60790.1 (Genbank) | URP |
| Escherichia coli MP021552.11 Taxon ID: 1116134 | 476034829 | EMU60386.1 (Genbank) | URP |
| Escherichia coli O127:H27 str. C43/90 Taxon ID: 1090929 | 472425136 | EMS07573.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. E112/10 Taxon ID: 1090928 | 472418823 | EMS01395.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. E92/11 Taxon ID: 1090927 | 472417180 | EMR99811.1 (Genbank) | URP |
| Escherichia coli ONT:H33 str. C48/93 Taxon ID: 1090930 | 472411466 | EMR94239.1 (Genbank) | URP |
| Escherichia coli SEPT362 Taxon ID: 1196033 | 449319546 | EMD09595.1 (Genbank) | URP |
| Escherichia coli S17 Taxon ID: 1227813 | 449319217 | EMD09272.1 (Genbank) | URP |
| Escherichia coli O08 Taxon ID: 1227814 | 449316974 | EMD07070.1 (Genbank) | URP |
| Escherichia coli APEC O78 Taxon ID: 1274814 | 443423080 | AGC87984.1 (Genbank) | URP |
| Escherichia coli J96 Taxon ID: 1206108 | 432348510 | ELL42960.1 (Genbank) | URP |
| Escherichia coli KTE94 Taxon ID: 1169368 | 431720559 | ELJ84586.1 (Genbank) | URP |
| Escherichia coli KTE95 Taxon ID: 1169369 | 431720133 | ELJ84167.1 (Genbank) | URP |
| Escherichia coli KTE90 Taxon ID: 1169367 | 431715074 | ELJ79243.1 (Genbank) | URP |
| Escherichia coli KTE85 Taxon ID: 1169364 | 431705318 | ELJ69915.1 (Genbank) | URP |
| Escherichia coli KTE88 Taxon ID: 1169366 | 431705174 | ELJ69772.1 (Genbank) | URP |
| Escherichia coli KTE232 Taxon ID: 1169365 | 431691631 | ELJ57089.1 (Genbank) | URP |
| Escherichia coli KTE180 Taxon ID: 1169404 | 431686833 | ELJ52389.1 (Genbank) | URP |
| Escherichia coli KTE179 Taxon ID: 1169403 | 431686686 | ELJ52246.1 (Genbank) | URP |
| Escherichia coli KTE176 Taxon ID: 1169401 | 431673120 | ELJ39350.1 (Genbank) | URP |
| Escherichia coli KTE168 Taxon ID: 1169399 | 431660749 | ELJ27612.1 (Genbank) | URP |
| Escherichia coli KTE166 Taxon ID: 1169397 | 431655655 | ELJ22686.1 (Genbank) | URP |
| Escherichia coli KTE163 Taxon ID: 1169396 | 431645856 | ELJ13400.1 (Genbank) | URP |
| Escherichia coli KTE157 Taxon ID: 1169394 | 431641776 | ELJ09510.1 (Genbank) | URP |
| Escherichia coli KTE150 Taxon ID: 1169392 | 431627501 | ELI95903.1 (Genbank) | URP |
| Escherichia coli KTE148 Taxon ID: 1169391 | 431626490 | ELI95039.1 (Genbank) | URP |
| Escherichia coli KTE145 Taxon ID: 1169390 | 431618336 | ELI87310.1 (Genbank) | URP |
| Escherichia coli KTE139 Taxon ID: 1169389 | 431614919 | ELI84053.1 (Genbank) | URP |
| Escherichia coli KTE138 Taxon ID: 1169388 | 431609574 | ELI78891.1 (Genbank) | URP |
| Escherichia coli KTE133 Taxon ID: 1169386 | 431601555 | ELI71071.1 (Genbank) | URP |
| Escherichia coli KTE131 Taxon ID: 1169385 | 431595821 | ELI65808.1 (Genbank) | URP |
| Escherichia coli KTE129 Taxon ID: 1169384 | 431587114 | ELI58495.1 (Genbank) | URP |
| Escherichia coli KTE125 Taxon ID: 1169382 | 431580743 | ELI53300.1 (Genbank) | URP |
| Escherichia coli KTE120 Taxon ID: 1169379 | 431563710 | ELI36908.1 (Genbank) | URP |
| Escherichia coli KTE117 Taxon ID: 1169378 | 431555375 | ELI29217.1 (Genbank) | URP |
| Escherichia coli KTE113 Taxon ID: 1169377 | 431550634 | ELI24623.1 (Genbank) | URP |
| Escherichia coli KTE112 Taxon ID: 1169376 | 431549735 | ELI23811.1 (Genbank) | URP |
| Escherichia coli KTE109 Taxon ID: 1169375 | 431542507 | ELI17674.1 (Genbank) | URP |
| Escherichia coli KTE106 Taxon ID: 1169374 | 431534432 | ELI10915.1 (Genbank) | URP |
| Escherichia coli KTE104 Taxon ID: 1169372 | 431529785 | ELI06480.1 (Genbank) | URP |
| Escherichia coli KTE229 Taxon ID: 1181775 | 431523191 | ELI00335.1 (Genbank) | URP |
| Escherichia coli KTE227 Taxon ID: 1181773 | 431513678 | ELH91760.1 (Genbank) | URP |
| Escherichia coli KTE215 Taxon ID: 1181761 | 431499127 | ELH78308.1 (Genbank) | URP |
| Escherichia coli KTE217 Taxon ID: 1181763 | 431495706 | ELH75292.1 (Genbank) | URP |
| Escherichia coli KTE211 Taxon ID: 1181757 | 431491205 | ELH70812.1 (Genbank) | URP |
| Escherichia coli KTE207 Taxon ID: 1181753 | 431480814 | ELH60530.1 (Genbank) | URP |
| Escherichia coli KTE202 Taxon ID: 1181748 | 431472656 | ELH52543.1 (Genbank) | URP |
| Escherichia coli KTE203 Taxon ID: 1181749 | 431470012 | ELH49936.1 (Genbank) | URP |
| Escherichia coli KTE197 Taxon ID: 1181743 | 431466922 | ELH46938.1 (Genbank) | URP |
| Escherichia coli KTE183 Taxon ID: 1181729 | 431462845 | ELH43052.1 (Genbank) | URP |
| Escherichia coli KTE196 Taxon ID: 1181742 | 431458342 | ELH38679.1 (Genbank) | URP |
| Escherichia coli KTE184 Taxon ID: 1181730 | 431452197 | ELH32646.1 (Genbank) | URP |
| Escherichia coli KTE175 Taxon ID: 1181725 | 431443058 | ELH24136.1 (Genbank) | URP |
| Escherichia coli KTE173 Taxon ID: 1181724 | 431441698 | ELH22806.1 (Genbank) | URP |
| Escherichia coli KTE194 Taxon ID: 1181740 | 431431917 | ELH13691.1 (Genbank) | URP |
| Escherichia coli KTE192 Taxon ID: 1181738 | 431425723 | ELH07791.1 (Genbank) | URP |
| Escherichia coli KTE165 Taxon ID: 1182735 | 431421858 | ELH04070.1 (Genbank) | URP |
| Escherichia coli KTE154 Taxon ID: 1182728 | 431419784 | ELH02124.1 (Genbank) | URP |
| Escherichia coli KTE147 Taxon ID: 1182726 | 431410439 | ELG93601.1 (Genbank) | URP |
| Escherichia coli KTE144 Taxon ID: 1182724 | 431399431 | ELG82838.1 (Genbank) | URP |
| Escherichia coli KTE140 Taxon ID: 1182720 | 431388343 | ELG72079.1 (Genbank) | URP |
| Escherichia coli KTE135 Taxon ID: 1182718 | 431374852 | ELG60197.1 (Genbank) | URP |
| Escherichia coli KTE123 Taxon ID: 1182712 | 431371495 | ELG57204.1 (Genbank) | URP |
| Escherichia coli KTE118 Taxon ID: 1182709 | 431367521 | ELG53998.1 (Genbank) | URP |
| Escherichia coli KTE115 Taxon ID: 1182707 | 431363659 | ELG50212.1 (Genbank) | URP |
| Escherichia coli KTE101 Taxon ID: 1182700 | 431362954 | ELG49532.1 (Genbank) | URP |
| Escherichia coli KTE91 Taxon ID: 1182694 | 431354839 | ELG41565.1 (Genbank) | URP |
| Escherichia coli KTE84 Taxon ID: 1182690 | 431347854 | ELG34731.1 (Genbank) | URP |
| Escherichia coli KTE79 Taxon ID: 1182686 | 431342775 | ELG29746.1 (Genbank) | URP |
| Escherichia coli KTE78 Taxon ID: 1182685 | 431339371 | ELG26433.1 (Genbank) | URP |
| Escherichia coli KTE65 Taxon ID: 1182672 | 431336618 | ELG23726.1 (Genbank) | URP |
| Escherichia coli KTE59 Taxon ID: 1182666 | 431326237 | ELG13599.1 (Genbank) | URP |
| Escherichia coli KTE50 Taxon ID: 1182657 | 431313935 | ELG01890.1 (Genbank) | URP |
| Escherichia coli KTE48 Taxon ID: 1182655 | 431310050 | ELF98243.1 (Genbank) | URP |
| Escherichia coli KTE46 Taxon ID: 1182653 | 431307995 | ELF96285.1 (Genbank) | URP |
| Escherichia coli KTE22 Taxon ID: 1169341 | 431301781 | ELF90982.1 (Genbank) | URP |
| Escherichia coli KTE29 Taxon ID: 1169348 | 431295984 | ELF85714.1 (Genbank) | URP |
| Escherichia coli KTE43 Taxon ID: 1169360 | 431291054 | ELF81577.1 (Genbank) | URP |
| Escherichia coli KTE23 Taxon ID: 1169342 | 431283949 | ELF74808.1 (Genbank) | URP |
| Escherichia coli KTE42 Taxon ID: 1169359 | 431281797 | ELF72696.1 (Genbank) | URP |
| Escherichia coli KTE45 Taxon ID: 1169362 | 431274468 | ELF65525.1 (Genbank) | URP |
| Escherichia coli KTE18 Taxon ID: 1169337 | 431273126 | ELF64224.1 (Genbank) | URP |
| Escherichia coli KTE17 Taxon ID: 1169336 | 431264585 | ELF56290.1 (Genbank) | URP |
| Escherichia coli KTE9 Taxon ID: 1169329 | 431261858 | ELF53881.1 (Genbank) | URP |
| Escherichia coli KTE8 Taxon ID: 1169328 | 431256107 | ELF49184.1 (Genbank) | URP |
| Escherichia coli KTE6 Taxon ID: 1169327 | 431248489 | ELF42683.1 (Genbank) | URP |
| Escherichia coli KTE169 Taxon ID: 1182736 | 431242927 | ELF37317.1 (Genbank) | URP |
| Escherichia coli KTE171 Taxon ID: 1182738 | 431242177 | ELF36598.1 (Genbank) | URP |
| Escherichia coli KTE162 Taxon ID: 1182734 | 431233424 | ELF29015.1 (Genbank) | URP |
| Escherichia coli KTE161 Taxon ID: 1182733 | 431226395 | ELF23560.1 (Genbank) | URP |
| Escherichia coli KTE156 Taxon ID: 1182730 | 431221063 | ELF18385.1 (Genbank) | URP |
| Escherichia coli KTE143 Taxon ID: 1182723 | 431219857 | ELF17246.1 (Genbank) | URP |
| Escherichia coli KTE142 Taxon ID: 1182722 | 431213448 | ELF11322.1 (Genbank) | URP |
| Escherichia coli KTE119 Taxon ID: 1182710 | 431209904 | ELF07971.1 (Genbank) | URP |
| Escherichia coli KTE111 Taxon ID: 1182706 | 431198687 | ELE97470.1 (Genbank) | URP |
| Escherichia coli KTE93 Taxon ID: 1182696 | 431190546 | ELE89945.1 (Genbank) | URP |
| Escherichia coli KTE86 Taxon ID: 1182691 | 431179700 | ELE79592.1 (Genbank) | URP |
| Escherichia coli KTE81 Taxon ID: 1182688 | 431170499 | ELE70692.1 (Genbank) | URP |
| Escherichia coli KTE77 Taxon ID: 1182684 | 431162752 | ELE63193.1 (Genbank) | URP |
| Escherichia coli KTE76 Taxon ID: 1182683 | 431158408 | ELE59007.1 (Genbank) | URP |
| Escherichia coli KTE75 Taxon ID: 1182682 | 431153647 | ELE54551.1 (Genbank) | URP |
| Escherichia coli KTE72 Taxon ID: 1182679 | 431148556 | ELE49847.1 (Genbank) | URP |
| Escherichia coli KTE66 Taxon ID: 1182673 | 431141722 | ELE43487.1 (Genbank) | URP |
| Escherichia coli KTE62 Taxon ID: 1182669 | 431130036 | ELE32145.1 (Genbank) | URP |
| Escherichia coli KTE58 Taxon ID: 1182665 | 431121132 | ELE24130.1 (Genbank) | URP |
| Escherichia coli KTE57 Taxon ID: 1182664 | 431115486 | ELE18989.1 (Genbank) | URP |
| Escherichia coli KTE56 Taxon ID: 1182663 | 431114454 | ELE17996.1 (Genbank) | URP |
| Escherichia coli KTE55 Taxon ID: 1182662 | 431107064 | ELE11252.1 (Genbank) | URP |
| Escherichia coli KTE51 Taxon ID: 1182658 | 431092778 | ELD98459.1 (Genbank) | URP |
| Escherichia coli KTE49 Taxon ID: 1182656 | 431090952 | ELD96703.1 (Genbank) | URP |
| Escherichia coli KTE47 Taxon ID: 1182654 | 431083229 | ELD89536.1 (Genbank) | URP |
| Escherichia coli KTE237 Taxon ID: 1181781 | 431079225 | ELD86195.1 (Genbank) | URP |
| Escherichia coli KTE236 Taxon ID: 1181780 | 431073852 | ELD81490.1 (Genbank) | URP |
| Escherichia coli KTE233 Taxon ID: 1181777 | 431063099 | ELD72356.1 (Genbank) | URP |
| Escherichia coli KTE224 Taxon ID: 1181770 | 431041209 | ELD51740.1 (Genbank) | URP |
| Escherichia coli KTE216 Taxon ID: 1181762 | 431028586 | ELD41630.1 (Genbank) | URP |
| Escherichia coli KTE213 Taxon ID: 1181759 | 431019737 | ELD33131.1 (Genbank) | URP |
| Escherichia coli KTE212 Taxon ID: 1181758 | 431015508 | ELD29063.1 (Genbank) | URP |
| Escherichia coli KTE210 Taxon ID: 1181756 | 431006389 | ELD21395.1 (Genbank) | URP |
| Escherichia coli KTE206 Taxon ID: 1181752 | 430997586 | ELD13847.1 (Genbank) | URP |
| Escherichia coli KTE205 Taxon ID: 1181751 | 430992989 | ELD09348.1 (Genbank) | URP |
| Escherichia coli KTE193 Taxon ID: 1181739 | 430979141 | ELC95927.1 (Genbank) | URP |
| Escherichia coli KTE191 Taxon ID: 1181737 | 430973362 | ELC90330.1 (Genbank) | URP |
| Escherichia coli KTE189 Taxon ID: 1181735 | 430966437 | ELC83845.1 (Genbank) | URP |
| Escherichia coli KTE178 Taxon ID: 1181726 | 430943605 | ELC63712.1 (Genbank) | URP |
| Escherichia coli KTE44 Taxon ID: 1169361 | 430938103 | ELC58346.1 (Genbank) | URP |
| Escherichia coli KTE28 Taxon ID: 1169347 | 430929072 | ELC49593.1 (Genbank) | URP |
| Escherichia coli KTE21 Taxon ID: 1169340 | 430918185 | ELC39224.1 (Genbank) | URP |
| Escherichia coli KTE25 Taxon ID: 1169344 | 430914721 | ELC35816.1 (Genbank) | URP |
| Escherichia coli KTE15 Taxon ID: 1169334 | 430907687 | ELC29185.1 (Genbank) | URP |
| Escherichia coli KTE16 Taxon ID: 1169335 | 430905530 | ELC27139.1 (Genbank) | URP |
| Escherichia coli KTE12 Taxon ID: 1169332 | 430897747 | ELC19941.1 (Genbank) | URP |
| Escherichia coli KTE5 Taxon ID: 1169326 | 430885821 | ELC08691.1 (Genbank) | URP |
| Escherichia coli KTE10 Taxon ID: 1169330 | 430884544 | ELC07483.1 (Genbank) | URP |
| Escherichia coli KTE4 Taxon ID: 1169325 | 430876278 | ELB99797.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. Ec12-0466 Taxon ID: 1240779 | 429458075 | EKZ93913.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. Ec11-9941 Taxon ID: 1240759 | 429451417 | EKZ87308.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. Ec12-0465 Taxon ID: 1240778 | 429447164 | EKZ83088.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. Ec11-6006 Taxon ID: 1240767 | 429438795 | EKZ74788.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. Ec11-5603 Taxon ID: 1240765 | 429436590 | EKZ72606.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. Ec11-4988 Taxon ID: 1240764 | 429432384 | EKZ68424.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. Ec11-5604 Taxon ID: 1240766 | 429428831 | EKZ64906.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. Ec11-4986 Taxon ID: 1240762 | 429420659 | EKZ56784.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. Ec11-4987 Taxon ID: 1240763 | 429413881 | EKZ50061.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. Ec11-9450 Taxon ID: 1240758 | 429409969 | EKZ46194.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. Ec11-9990 Taxon ID: 1240760 | 429406064 | EKZ42325.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. Ec11-4984 Taxon ID: 1240761 | 429405528 | EKZ41794.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. 11-04080 Taxon ID: 1240776 | 429391164 | EKZ27569.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. 11-03943 Taxon ID: 1240777 | 429379461 | EKZ15961.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. 11-03439 Taxon ID: 1240775 | 429378597 | EKZ15105.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. 11-02281 Taxon ID: 1240772 | 429376288 | EKZ12817.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. 11-02913 Taxon ID: 1240774 | 429375131 | EKZ11669.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. 11-02318 Taxon ID: 1240773 | 429364282 | EKZ00902.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. 11-02033-1 Taxon ID: 1240769 | 429361642 | EKY98295.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. 11-02093 Taxon ID: 1240771 | 429361335 | EKY97990.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. 11-02030 Taxon ID: 1240768 | 429359004 | EKY95670.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. 11-02092 Taxon ID: 1240770 | 429348258 | EKY85028.1 (Genbank) | URP |
| Escherichia coli O111:H11 str. CFSAN001630 Taxon ID: 1232151 | 421937594 | EKT95203.1 (Genbank) | URP |
| Escherichia coli O26:H11 str. CFSAN001629 Taxon ID: 1232150 | 421933102 | EKT90896.1 (Genbank) | URP |
| Escherichia coli 8.0569 Taxon ID: 1005464 | 408568509 | EKK44540.1 (Genbank) | URP |
| Escherichia coli 8.0566 Taxon ID: 1005462 | 408567706 | EKK43760.1 (Genbank) | URP |
| Escherichia coli AD30 Taxon ID: 1169664 | 408460433 | EKJ84212.1 (Genbank) | URP |
| Escherichia coli 0.1288 Taxon ID: 1005442 | 408343253 | EKJ57657.1 (Genbank) | URP |
| Escherichia coli EC1865 Taxon ID: 1005550 | 408295564 | EKJ13869.1 (Genbank) | URP |
| Escherichia coli N1 Taxon ID: 1005567 | 408227879 | EKI51448.1 (Genbank) | URP |
| Escherichia coli 3006 Taxon ID: 1005565 | 408213030 | EKI37534.1 (Genbank) | URP |
| Escherichia coli 07798 Taxon ID: 1005566 | 408212764 | EKI37277.1 (Genbank) | URP |
| Escherichia coli TW00353 Taxon ID: 1005564 | 408201389 | EKI26544.1 (Genbank) | URP |
| Escherichia coli ARS4.2123 Taxon ID: 1005563 | 408200543 | EKI25720.1 (Genbank) | URP |
| Escherichia coli TW15901 Taxon ID: 1005562 | 408192944 | EKI18503.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. 2009EL-2071 Taxon ID: 1133853 | 407066448 | AFS87495.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. 2011C-3493 Taxon ID: 1133852 | 407053222 | AFS73273.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. 2009EL-2050 Taxon ID: 1134782 | 406776653 | AFS56077.1 (Genbank) | URP |
| Shigella flexneri 1485-80 Taxon ID: 766155 | 404337957 | EJZ64405.1 (Genbank) | URP |
| Shigella sonnei str. Moseley Taxon ID: 754077 | 397899130 | EJL15506.1 (Genbank) | URP |
| Escherichia coli STEC_O31 Taxon ID: 754081 | 397785065 | EJK95918.1 (Genbank) | URP |
| Escherichia coli O26:H11 str. CVM10030 Taxon ID: 1165952 | 394430590 | EJF02904.1 (Genbank) | URP |
| Escherichia coli O26:H11 str. CVM9952 Taxon ID: 1163738 | 394421284 | EJE94760.1 (Genbank) | URP |
| Escherichia coli O26:H11 str. CVM10021 Taxon ID: 1165950 | 394411219 | EJE85490.1 (Genbank) | URP |
| Escherichia coli O111:H11 str. CVM9455 Taxon ID: 1165939 | 394400229 | EJE76165.1 (Genbank) | URP |
| Escherichia coli O111:H11 str. CVM9553 Taxon ID: 1165947 | 394398243 | EJE74431.1 (Genbank) | URP |
| Escherichia coli O26:H11 str. CVM10224 Taxon ID: 1165953 | 394396699 | EJE73031.1 (Genbank) | URP |
| Escherichia coli EPEC C342-62 Taxon ID: 766166 | 391312030 | EIQ69653.1 (Genbank) | URP |
| Escherichia coli EPECa12 Taxon ID: 670896 | 391304857 | EIQ62659.1 (Genbank) | URP |
| Shigella dysenteriae 225-75 Taxon ID: 766143 | 391299858 | EIQ57797.1 (Genbank) | URP |
| Shigella sonnei 4822-66 Taxon ID: 766164 | 391293585 | EIQ51844.1 (Genbank) | URP |
| Shigella sonnei 3233-85 Taxon ID: 766163 | 391283880 | EIQ42489.1 (Genbank) | URP |
| Shigella sonnei 3226-85 Taxon ID: 766162 | 391281287 | EIQ39939.1 (Genbank) | URP |
| Shigella boydii 4444-74 Taxon ID: 766140 | 391277266 | EIQ36016.1 (Genbank) | URP |
| Shigella boydii 965-58 Taxon ID: 766138 | 391268909 | EIQ27828.1 (Genbank) | URP |
| Shigella flexneri K-315 Taxon ID: 766150 | 391260114 | EIQ19179.1 (Genbank) | URP |
| Shigella flexneri CCH060 Taxon ID: 754091 | 391250191 | EIQ09414.1 (Genbank) | URP |
| Escherichia coli MT8 Taxon ID: 752790 | 388415965 | EIL75872.1 (Genbank) | URP |
| Escherichia coli HM605 Taxon ID: 752789 | 388410502 | EIL70726.1 (Genbank) | URP |
| Escherichia coli 75 Taxon ID: 752788 | 388408645 | EIL68986.1 (Genbank) | URP |
| Escherichia coli 541-1 Taxon ID: 752786 | 388398928 | EIL59739.1 (Genbank) | URP |
| Escherichia coli KD1 Taxon ID: 752783 | 388385715 | EIL47386.1 (Genbank) | URP |
| Escherichia coli KD2 Taxon ID: 752784 | 388384875 | EIL46581.1 (Genbank) | URP |
| Escherichia coli O26:H11 str. CVM9942 Taxon ID: 1165949 | 388379687 | EIL42334.1 (Genbank) | URP |
| Escherichia coli O26:H11 str. CVM10026 Taxon ID: 1165951 | 388378693 | EIL41407.1 (Genbank) | URP |
| Escherichia coli O111:H11 str. CVM9545 Taxon ID: 1163391 | 388348459 | EIL14054.1 (Genbank) | URP |
| Escherichia coli O103:H25 str. CVM9340 Taxon ID: 1165940 | 388336172 | EIL02719.1 (Genbank) | URP |
| Escherichia coli O111:H11 str. CVM9534 Taxon ID: 1165946 | 388334450 | EIL01041.1 (Genbank) | URP |
| Escherichia coli O103:H2 str. CVM9450 Taxon ID: 1165941 | 388333720 | EIL00337.1 (Genbank) | URP |
| Escherichia coli 900105 (10e) Taxon ID: 869695 | 386256702 | EIJ12196.1 (Genbank) | URP |
| Escherichia coli B41 Taxon ID: 869694 | 386252944 | EIJ02634.1 (Genbank) | URP |
| Escherichia coli TW07793 Taxon ID: 869693 | 386249965 | EII96134.1 (Genbank) | URP |
| Escherichia coli 3003 Taxon ID: 869692 | 386245149 | EII86879.1 (Genbank) | URP |
| Escherichia coli 3.2303 Taxon ID: 869691 | 386239173 | EII76107.1 (Genbank) | URP |
| Escherichia coli 2.4168 Taxon ID: 869690 | 386236391 | EII68367.1 (Genbank) | URP |
| Escherichia coli 3.3884 Taxon ID: 869689 | 386227812 | EII55168.1 (Genbank) | URP |
| Escherichia coli 2.3916 Taxon ID: 869688 | 386224810 | EII47145.1 (Genbank) | URP |
| Escherichia coli 4.0967 Taxon ID: 869687 | 386218741 | EII35224.1 (Genbank) | URP |
| Escherichia coli 9.0111 Taxon ID: 869686 | 386213730 | EII24155.1 (Genbank) | URP |
| Escherichia coli 5.0959 Taxon ID: 869684 | 386204145 | EII08656.1 (Genbank) | URP |
| Escherichia coli 96.154 Taxon ID: 869683 | 386201787 | EII00778.1 (Genbank) | URP |
| Escherichia coli 93.0624 Taxon ID: 869680 | 386183133 | EIH65884.1 (Genbank) | URP |
| Escherichia coli 3.2608 Taxon ID: 869679 | 386178012 | EIH55491.1 (Genbank) | URP |
| Escherichia coli 99.0741 Taxon ID: 869678 | 386172778 | EIH44792.1 (Genbank) | URP |
| Escherichia coli 96.0497 Taxon ID: 869677 | 386168554 | EIH35070.1 (Genbank) | URP |
| Escherichia coli 1.2264 Taxon ID: 869675 | 386163844 | EIH25639.1 (Genbank) | URP |
| Escherichia coli 97.0259 Taxon ID: 869672 | 386156104 | EIH12451.1 (Genbank) | URP |
| Escherichia coli 5.0588 Taxon ID: 869671 | 386153186 | EIH04475.1 (Genbank) | URP |
| Escherichia coli 1.2741 Taxon ID: 869669 | 386140612 | EIG81764.1 (Genbank) | URP |
| Escherichia sp. 4_1_40B Taxon ID: 457401 | 386121723 | EIG70338.1 (Genbank) | URP |
| Escherichia coli B799 Taxon ID: 550682 | 385711602 | EIG48560.1 (Genbank) | URP |
| Escherichia coli H730 Taxon ID: 656413 | 385707220 | EIG44252.1 (Genbank) | URP |
| Escherichia coli M919 Taxon ID: 656421 | 385537110 | EIF83993.1 (Genbank) | URP |
| Escherichia coli O32:H37 str. P4 Taxon ID: 1167694 | 385156948 | EIF18942.1 (Genbank) | URP |
| Escherichia coli AI27 Taxon ID: 1089445 | 384473440 | EIE57481.1 (Genbank) | URP |
| Escherichia coli J53 Taxon ID: 1144303 | 384379757 | EIE37624.1 (Genbank) | URP |
| Escherichia coli W26 Taxon ID: 1090926 | 383475446 | EID67406.1 (Genbank) | URP |
| Escherichia coli W Taxon ID: 566546 | 383406099 | AFH12342.1 (Genbank) | URP |
| Escherichia coli KO11 Taxon ID: 595495 | 383392064 | AFH17022.1 (Genbank) | URP |
| Escherichia coli P12b Taxon ID: 910348 | 383103950 | AFG41459.1 (Genbank) | URP |
| Escherichia coli SCI-07 Taxon ID: 1144302 | 380347562 | EIA35849.1 (Genbank) | URP |
| Escherichia coli DEC15E Taxon ID: 868210 | 378259103 | EHY18919.1 (Genbank) | URP |
| Escherichia coli DEC15D Taxon ID: 868209 | 378254460 | EHY14324.1 (Genbank) | URP |
| Escherichia coli DEC15C Taxon ID: 868208 | 378247496 | EHY07415.1 (Genbank) | URP |
| Escherichia coli DEC15B Taxon ID: 868207 | 378243741 | EHY03687.1 (Genbank) | URP |
| Escherichia coli DEC15A Taxon ID: 868206 | 378236829 | EHX96868.1 (Genbank) | URP |
| Escherichia coli DEC14D Taxon ID: 868204 | 378231657 | EHX91768.1 (Genbank) | URP |
| Escherichia coli DEC14C Taxon ID: 868203 | 378227429 | EHX87601.1 (Genbank) | URP |
| Escherichia coli DEC14B Taxon ID: 868202 | 378219173 | EHX79442.1 (Genbank) | URP |
| Escherichia coli DEC14A Taxon ID: 868201 | 378217595 | EHX77874.1 (Genbank) | URP |
| Escherichia coli DEC13E Taxon ID: 868200 | 378212344 | EHX72667.1 (Genbank) | URP |
| Escherichia coli DEC13D Taxon ID: 868199 | 378202788 | EHX63215.1 (Genbank) | URP |
| Escherichia coli DEC13C Taxon ID: 868198 | 378200309 | EHX60765.1 (Genbank) | URP |
| Escherichia coli DEC13B Taxon ID: 868197 | 378199885 | EHX60344.1 (Genbank) | URP |
| Escherichia coli DEC12E Taxon ID: 868195 | 378187867 | EHX48478.1 (Genbank) | URP |
| Escherichia coli DEC13A Taxon ID: 868196 | 378185612 | EHX46237.1 (Genbank) | URP |
| Escherichia coli DEC12D Taxon ID: 868194 | 378181011 | EHX41688.1 (Genbank) | URP |
| Escherichia coli DEC12C Taxon ID: 868193 | 378170262 | EHX31148.1 (Genbank) | URP |
| Escherichia coli DEC12A Taxon ID: 868191 | 378168501 | EHX29405.1 (Genbank) | URP |
| Escherichia coli DEC12B Taxon ID: 868192 | 378165033 | EHX25974.1 (Genbank) | URP |
| Escherichia coli DEC11E Taxon ID: 868190 | 378158000 | EHX19031.1 (Genbank) | URP |
| Escherichia coli DEC11C Taxon ID: 868188 | 378150850 | EHX11965.1 (Genbank) | URP |
| Escherichia coli DEC11D Taxon ID: 868189 | 378148345 | EHX09485.1 (Genbank) | URP |
| Escherichia coli DEC11B Taxon ID: 868187 | 378140564 | EHX01787.1 (Genbank) | URP |
| Escherichia coli DEC10F Taxon ID: 868185 | 378130561 | EHW91925.1 (Genbank) | URP |
| Escherichia coli DEC11A Taxon ID: 868186 | 378130246 | EHW91616.1 (Genbank) | URP |
| Escherichia coli DEC10E Taxon ID: 868184 | 378127751 | EHW89139.1 (Genbank) | URP |
| Escherichia coli DEC10D Taxon ID: 868183 | 378116237 | EHW77770.1 (Genbank) | URP |
| Escherichia coli DEC10C Taxon ID: 868182 | 378110904 | EHW72498.1 (Genbank) | URP |
| Escherichia coli DEC10B Taxon ID: 868181 | 378104989 | EHW66637.1 (Genbank) | URP |
| Escherichia coli DEC10A Taxon ID: 868180 | 378099465 | EHW61171.1 (Genbank) | URP |
| Escherichia coli DEC9E Taxon ID: 868179 | 378094148 | EHW55950.1 (Genbank) | URP |
| Escherichia coli DEC9D Taxon ID: 868178 | 378090298 | EHW52138.1 (Genbank) | URP |
| Escherichia coli DEC9C Taxon ID: 868177 | 378083924 | EHW45855.1 (Genbank) | URP |
| Escherichia coli DEC9B Taxon ID: 868176 | 378076654 | EHW38658.1 (Genbank) | URP |
| Escherichia coli DEC9A Taxon ID: 868175 | 378073270 | EHW35323.1 (Genbank) | URP |
| Escherichia coli DEC8D Taxon ID: 868173 | 378060798 | EHW22986.1 (Genbank) | URP |
| Escherichia coli DEC8C Taxon ID: 868172 | 378053442 | EHW15742.1 (Genbank) | URP |
| Escherichia coli DEC7E Taxon ID: 868169 | 378037916 | EHW00438.1 (Genbank) | URP |
| Escherichia coli DEC7B Taxon ID: 868166 | 378032120 | EHV94702.1 (Genbank) | URP |
| Escherichia coli DEC7D Taxon ID: 868168 | 378029256 | EHV91872.1 (Genbank) | URP |
| Escherichia coli DEC7C Taxon ID: 868167 | 378023450 | EHV86127.1 (Genbank) | URP |
| Escherichia coli DEC7A Taxon ID: 868165 | 378015230 | EHV78127.1 (Genbank) | URP |
| Escherichia coli DEC6E Taxon ID: 868164 | 378009772 | EHV72726.1 (Genbank) | URP |
| Escherichia coli DEC6D Taxon ID: 868163 | 378007548 | EHV70517.1 (Genbank) | URP |
| Escherichia coli DEC6C Taxon ID: 868162 | 377995849 | EHV58960.1 (Genbank) | URP |
| Escherichia coli DEC6A Taxon ID: 868160 | 377993980 | EHV57111.1 (Genbank) | URP |
| Escherichia coli DEC6B Taxon ID: 868161 | 377992410 | EHV55557.1 (Genbank) | URP |
| Escherichia coli DEC2E Taxon ID: 868142 | 377890709 | EHU55166.1 (Genbank) | URP |
| Escherichia coli DEC2D Taxon ID: 868141 | 377879635 | EHU44207.1 (Genbank) | URP |
| Escherichia coli DEC2C Taxon ID: 868140 | 377876983 | EHU41581.1 (Genbank) | URP |
| Escherichia coli DEC2B Taxon ID: 868139 | 377873348 | EHU37985.1 (Genbank) | URP |
| Escherichia coli DEC2A Taxon ID: 868138 | 377863730 | EHU28535.1 (Genbank) | URP |
| Escherichia coli DEC1E Taxon ID: 868137 | 377859449 | EHU24280.1 (Genbank) | URP |
| Escherichia coli DEC1D Taxon ID: 868136 | 377856396 | EHU21256.1 (Genbank) | URP |
| Escherichia coli DEC1B Taxon ID: 868134 | 377846674 | EHU11681.1 (Genbank) | URP |
| Escherichia coli DEC1C Taxon ID: 868135 | 377844272 | EHU09309.1 (Genbank) | URP |
| Escherichia coli DEC1A Taxon ID: 868133 | 377843421 | EHU08461.1 (Genbank) | URP |
| Escherichia coli 4_1_47FAA Taxon ID: 1127356 | 373245520 | EHP64988.1 (Genbank) | URP |
| Escherichia coli H397 Taxon ID: 656398 | 371615228 | EHO03656.1 (Genbank) | URP |
| Escherichia coli E101 Taxon ID: 550685 | 371612209 | EHO00725.1 (Genbank) | URP |
| Escherichia coli B093 Taxon ID: 550674 | 371607191 | EHN95769.1 (Genbank) | URP |
| Escherichia coli TA124 Taxon ID: 656435 | 371597535 | EHN86356.1 (Genbank) | URP |
| Escherichia coli cloneA_i1 Taxon ID: 993513 | 355350895 | EHG00090.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. 11-4632 C4 Taxon ID: 1068620 | 354917419 | EHF77385.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. 11-4632 C3 Taxon ID: 1068619 | 354915185 | EHF75165.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. 11-4632 C5 Taxon ID: 1068621 | 354912229 | EHF72230.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. 11-4632 C2 Taxon ID: 1068618 | 354898461 | EHF58615.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. 11-4623 Taxon ID: 1068616 | 354897097 | EHF57258.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. 11-4632 C1 Taxon ID: 1068617 | 354894305 | EHF54501.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. 11-4522 Taxon ID: 1068615 | 354893354 | EHF53558.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. 11-4404 Taxon ID: 1068614 | 354889124 | EHF49377.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. 11-3677 Taxon ID: 1048334 | 354880335 | EHF40671.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. 09-7901 Taxon ID: 1048266 | 354873651 | EHF34028.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. 04-8351 Taxon ID: 1048265 | 354868755 | EHF29168.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. C227-11 Taxon ID: 1048254 | 354867654 | EHF28076.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. C236-11 Taxon ID: 1048256 | 354862370 | EHF22808.1 (Genbank) | URP |
| Escherichia coli O7:K1 str. CE10 Taxon ID: 1072459 | 349738991 | AEQ13697.1 (Genbank) | URP |
| Escherichia coli TX1999 Taxon ID: 670903 | 345393272 | EGX23050.1 (Genbank) | URP |
| Escherichia coli STEC_S1191 Taxon ID: 754090 | 345387029 | EGX16858.1 (Genbank) | URP |
| Escherichia coli STEC_H.1.8 Taxon ID: 754088 | 345377433 | EGX09365.1 (Genbank) | URP |
| Escherichia coli G58-1 Taxon ID: 754078 | 345375815 | EGX07762.1 (Genbank) | URP |
| Escherichia coli STEC_MHI813 Taxon ID: 754089 | 345372563 | EGX04527.1 (Genbank) | URP |
| Escherichia coli STEC_EH250 Taxon ID: 754087 | 345362177 | EGW94334.1 (Genbank) | URP |
| Escherichia coli 3030-1 Taxon ID: 754080 | 345352349 | EGW84598.1 (Genbank) | URP |
| Escherichia coli STEC_94C Taxon ID: 754083 | 345350837 | EGW83112.1 (Genbank) | URP |
| Escherichia coli STEC_B2F1 Taxon ID: 754084 | 345337791 | EGW70223.1 (Genbank) | URP |
| Escherichia coli XH001 Taxon ID: 1069495 | 344194583 | EGV48656.1 (Genbank) | URP |
| Escherichia coli MS 79-10 Taxon ID: 796392 | 342930490 | EGU99212.1 (Genbank) | URP |
| Escherichia coli XH140A Taxon ID: 1068608 | 342364541 | EGU28641.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. C227-11 Taxon ID: 1048254 | 341916433 | EGT66050.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. LB226692 Taxon ID: 1040638 | 340739363 | EGR73598.1 (Genbank) | URP |
| Escherichia coli O104:H4 str. 01-09591 Taxon ID: 1042803 | 340733456 | EGR62587.1 (Genbank) | URP |
| Escherichia coli UMNF18 Taxon ID: 1050617 | 339416064 | AEJ57736.1 (Genbank) | URP |
| Escherichia coli NA114 Taxon ID: 1033813 | 333970625 | AEG37430.1 (Genbank) | URP |
| Escherichia coli UMNK88 Taxon ID: 696406 | 332344389 | AEE57723.1 (Genbank) | URP |
| Shigella sp. D9 Taxon ID: 556266 | 332100682 | EGJ04028.1 (Genbank) | URP |
| Shigella boydii 3594-74 Taxon ID: 766139 | 332092803 | EGI97872.1 (Genbank) | URP |
| Escherichia coli H299 Taxon ID: 656393 | 331079117 | EGI50319.1 (Genbank) | URP |
| Escherichia coli H591 Taxon ID: 656408 | 331074968 | EGI46288.1 (Genbank) | URP |
| Escherichia coli TA280 Taxon ID: 656444 | 331069245 | EGI40637.1 (Genbank) | URP |
| Escherichia coli TA271 Taxon ID: 656443 | 331064457 | EGI36368.1 (Genbank) | URP |
| Escherichia coli TA143 Taxon ID: 656437 | 331059879 | EGI31856.1 (Genbank) | URP |
| Escherichia coli TA206 Taxon ID: 656440 | 331054327 | EGI26354.1 (Genbank) | URP |
| Escherichia coli M718 Taxon ID: 656419 | 331048794 | EGI20870.1 (Genbank) | URP |
| Escherichia coli M605 Taxon ID: 656417 | 331043074 | EGI15214.1 (Genbank) | URP |
| Escherichia coli H736 Taxon ID: 656414 | 331039935 | EGI12155.1 (Genbank) | URP |
| Escherichia coli AA86 Taxon ID: 1004153 | 330912292 | EGH40802.1 (Genbank) | URP |
| Escherichia coli STEC_7v Taxon ID: 754082 | 327252224 | EGE63896.1 (Genbank) | URP |
| Escherichia coli E1167 Taxon ID: 656372 | 324118139 | EGC12036.1 (Genbank) | URP |
| Escherichia coli MS 117-3 Taxon ID: 749539 | 324020077 | EGB89296.1 (Genbank) | URP |
| Escherichia coli MS 60-1 Taxon ID: 749530 | 324011226 | EGB80445.1 (Genbank) | URP |
| Escherichia coli MS 57-2 Taxon ID: 749529 | 324008525 | EGB77744.1 (Genbank) | URP |
| Escherichia coli TW10509 Taxon ID: 656449 | 323977300 | EGB72386.1 (Genbank) | URP |
| Escherichia coli TA007 Taxon ID: 656429 | 323971070 | EGB66318.1 (Genbank) | URP |
| Escherichia coli M863 Taxon ID: 656420 | 323967965 | EGB63377.1 (Genbank) | URP |
| Escherichia coli H489 Taxon ID: 656404 | 323961333 | EGB56945.1 (Genbank) | URP |
| Escherichia coli H263 Taxon ID: 656390 | 323955758 | EGB51516.1 (Genbank) | URP |
| Escherichia coli H252 Taxon ID: 656388 | 323949177 | EGB45068.1 (Genbank) | URP |
| Escherichia coli H120 Taxon ID: 656383 | 323944739 | EGB40806.1 (Genbank) | URP |
| Escherichia coli E482 Taxon ID: 550687 | 323941221 | EGB37406.1 (Genbank) | URP |
| Escherichia coli E1520 Taxon ID: 656374 | 323936410 | EGB32700.1 (Genbank) | URP |
| Escherichia coli KO11 Taxon ID: 595495 | 323377583 | ADX49851.1 (Genbank) | URP |
| Escherichia coli RN587/1 Taxon ID: 670899 | 323188365 | EFZ73657.1 (Genbank) | URP |
| Escherichia coli 1357 Taxon ID: 670905 | 323184422 | EFZ69797.1 (Genbank) | URP |
| Escherichia coli LT-68 Taxon ID: 670890 | 323170210 | EFZ55863.1 (Genbank) | URP |
| Shigella sonnei 53G Taxon ID: 216599 | 323169039 | EFZ54716.1 (Genbank) | URP |
| Escherichia coli E128010 Taxon ID: 670894 | 323159387 | EFZ45372.1 (Genbank) | URP |
| Escherichia coli EPECa14 Taxon ID: 670893 | 323156172 | EFZ42331.1 (Genbank) | URP |
| Escherichia coli EC4100B Taxon ID: 945434 | 320200081 | EFW74670.1 (Genbank) | URP |
| Escherichia coli WV_060327 Taxon ID: 945433 | 320196354 | EFW70978.1 (Genbank) | URP |
| Shigella flexneri CDC 796-83 Taxon ID: 945360 | 320185217 | EFW59997.1 (Genbank) | URP |
| Shigella boydii ATCC 9905 Taxon ID: 932676 | 320180507 | EFW55438.1 (Genbank) | URP |
| Shigella dysenteriae CDC 74-1112 Taxon ID: 941429 | 320175080 | EFW50193.1 (Genbank) | URP |
| Escherichia coli 3431 Taxon ID: 670892 | 315615776 | EFU96408.1 (Genbank) | URP |
| Escherichia coli MS 16-3 Taxon ID: 749542 | 315300476 | EFU59705.1 (Genbank) | URP |
| Escherichia coli MS 110-3 Taxon ID: 749536 | 315288069 | EFU47469.1 (Genbank) | URP |
| Escherichia coli MS 85-1 Taxon ID: 679202 | 315256537 | EFU36505.1 (Genbank) | URP |
| Escherichia coli W Taxon ID: 566546 | 315061836 | ADT76163.1 (Genbank) | URP |
| Escherichia coli O83:H1 str. NRG 857C Taxon ID: 685038 | 312947094 | ADR27921.1 (Genbank) | URP |
| Escherichia coli 2362-75 Taxon ID: 670897 | 312289853 | EFR17741.1 (Genbank) | URP |
| Shigella dysenteriae 1617 Taxon ID: 754093 | 308927102 | EFP72576.1 (Genbank) | URP |
| Escherichia coli MS 145-7 Taxon ID: 679204 | 308121892 | EFO59154.1 (Genbank) | URP |
| Escherichia coli UM146 Taxon ID: 869729 | 307625927 | ADN70231.1 (Genbank) | URP |
| Escherichia coli W Taxon ID: 566546 | 306907411 | EFN37915.1 (Genbank) | URP |
| Escherichia coli NC101 Taxon ID: 753642 | 305851807 | EFM52259.1 (Genbank) | URP |
| Escherichia coli MS 146-1 Taxon ID: 749540 | 301073659 | EFK88465.1 (Genbank) | URP |
| Escherichia coli MS 78-1 Taxon ID: 749532 | 300843621 | EFK71381.1 (Genbank) | URP |
| Escherichia coli MS 124-1 Taxon ID: 679205 | 300841809 | EFK69569.1 (Genbank) | URP |
| Escherichia coli MS 107-1 Taxon ID: 679207 | 300529701 | EFK50763.1 (Genbank) | URP |
| Escherichia coli MS 119-7 Taxon ID: 679206 | 300526566 | EFK47635.1 (Genbank) | URP |
| Escherichia coli MS 187-1 Taxon ID: 749547 | 300461963 | EFK25456.1 (Genbank) | URP |
| Escherichia coli MS 21-1 Taxon ID: 749527 | 300454928 | EFK18421.1 (Genbank) | URP |
| Escherichia coli MS 116-1 Taxon ID: 749538 | 300448975 | EFK12595.1 (Genbank) | URP |
| Escherichia coli MS 182-1 Taxon ID: 749545 | 300416890 | EFK00201.1 (Genbank) | URP |
| Escherichia coli MS 84-1 Taxon ID: 749533 | 300403741 | EFJ87279.1 (Genbank) | URP |
| Escherichia coli MS 69-1 Taxon ID: 749531 | 300396418 | EFJ79956.1 (Genbank) | URP |
| Escherichia coli MS 175-1 Taxon ID: 749544 | 300314505 | EFJ64289.1 (Genbank) | URP |
| Escherichia coli MS 200-1 Taxon ID: 749550 | 300304052 | EFJ58572.1 (Genbank) | URP |
| Escherichia coli MS 196-1 Taxon ID: 749548 | 299881276 | EFI89487.1 (Genbank) | URP |
| Escherichia coli IHE3034 Taxon ID: 714962 | 294492482 | ADE91238.1 (Genbank) | URP |
| Escherichia coli B354 Taxon ID: 550677 | 291471399 | EFF13883.1 (Genbank) | URP |
| Escherichia coli B088 Taxon ID: 550672 | 291323700 | EFE63128.1 (Genbank) | URP |
| Escherichia coli DH1 Taxon ID: 536056 | 260448403 | ACX38825.1 (Genbank) | URP |
| Escherichia coli BL21(DE3) Taxon ID: 469008 | 253978559 | ACT44229.1 (Genbank) | URP |
| Escherichia coli B str. REL606 Taxon ID: 413997 | 253974392 | ACT40063.1 (Genbank) | URP |
| Escherichia coli 'BL21-Gold(DE3)pLysS AG' Taxon ID: 866768 | 253323633 | ACT28235.1 (Genbank) | |
| Escherichia coli BW2952 Taxon ID: 595496 | 238862352 | ACR64350.1 (Genbank) | URP |
| Escherichia sp. 3_2_53FAA Taxon ID: 469598 | 226901393 | EEH87652.1 (Genbank) | URP |
| Escherichia coli 101-1 Taxon ID: 358709 | 194423369 | EDX39360.1 (Genbank) | URP |
| Escherichia coli B171 Taxon ID: 344601 | 194414649 | EDX30921.1 (Genbank) | URP |
| Escherichia coli E22 Taxon ID: 340185 | 192929344 | EDV82953.1 (Genbank) | URP |
| Escherichia coli F11 Taxon ID: 340197 | 190907091 | EDV66691.1 (Genbank) | URP |
| Escherichia coli B7A Taxon ID: 340184 | 190902278 | EDV62018.1 (Genbank) | URP |
| Escherichia coli 53638 Taxon ID: 344610 | 188487011 | EDU62114.1 (Genbank) | URP |
| Shigella boydii CDC 3083-94 Taxon ID: 344609 | 187428360 | ACD07634.1 (Genbank) | URP |
| Escherichia coli SMS-3-5 Taxon ID: 439855 | 170521524 | ACB19702.1 (Genbank) | URP |
| Escherichia coli str. K-12 substr. DH10B Taxon ID: 316385 | 169889962 | ACB03669.1 (Genbank) | URP |
| Escherichia coli ATCC 8739 Taxon ID: 481805 | 169754128 | ACA76827.1 (Genbank) | URP |
| Escherichia coli E24377A Taxon ID: 331111 | 157079868 | ABV19576.1 (Genbank) | URP |
| Escherichia coli APEC O1 Taxon ID: 405955 | 115513870 | ABJ01945.1 (Genbank) | URP |
| Escherichia coli 536 Taxon ID: 362663 | 110344274 | ABG70511.1 (Genbank) | URP |
| Escherichia coli UTI89 Taxon ID: 364106 | 91073417 | ABE08298.1 (Genbank) | URP |
| Shigella boydii Sb227 Taxon ID: 300268 | 81246377 | ABB67085.1 (Genbank) | URP |
| Shigella dysenteriae Sd197 Taxon ID: 300267 | 81242050 | ABB62760.1 (Genbank) | URP |
| Shigella sonnei Ss046 Taxon ID: 300269 | 73856520 | AAZ89227.1 (Genbank) | URP |
| Escherichia coli str. K-12 substr. MG1655 Taxon ID: 511145 | 1788865 | AAC75570.1 (Genbank) | URP |
| Escherichia coli Taxon ID: 562 | 493519 | AAA21359.1 (Genbank) | URP |
| Escherichia coli K-12 Taxon ID: 83333 | 802135902 | PRP URP | |
| Escherichia coli Taxon ID: 562 | 777220915 | URP | |
| Escherichia coli Taxon ID: 562 | 745372794 | URP | |
| Escherichia coli O26:H11 Taxon ID: 244319 | 740527055 | ||
| Escherichia coli Taxon ID: 562 | 687677650 | URP | |
| Escherichia coli Taxon ID: 562 | 687673365 | URP | |
| Escherichia coli Taxon ID: 562 | 678294220 | URP | |
| Trichuris trichiura Taxon ID: 36087 | 669218925 | URP | |
| Escherichia coli Taxon ID: 562 | 665441854 | URP | |
| Escherichia coli D6-113.11 Taxon ID: 1400022 | 665436865 | URP | |
| Escherichia coli ST131 Taxon ID: 1359206 | 641685337 | ||
| Escherichia coli D6-117.29 Taxon ID: 1400023 | 633887131 | URP | |
| Escherichia coli D6-113.11 Taxon ID: 1400022 | 633878048 | URP | |
| Escherichia coli Taxon ID: 562 | 633877451 | URP | |
| Escherichia coli IS29 Taxon ID: 1432549 | 572156338 | URP | |
| Escherichia coli IS35 Taxon ID: 1432551 | 571240973 | URP | |
| Escherichia coli IS9 Taxon ID: 1411645 | 571220397 | URP | |
| Klebsiella pneumoniae IS22 Taxon ID: 1432547 | 571214135 | ||
| Escherichia coli IS25 Taxon ID: 1432548 | 571177694 | URP | |
| Escherichia coli IS5 Taxon ID: 1433129 | 568394620 | URP | |
| Escherichia coli IS1 Taxon ID: 1433131 | 568389830 | URP | |
| Escherichia coli str. K-12 substr. MC4100 Taxon ID: 1403831 | 557272792 | URP | |
| Escherichia coli PMV-1 Taxon ID: 1382700 | 544343517 | URP | |
| Escherichia coli CAG:4 Taxon ID: 1263075 | 524544548 | URP | |
| Escherichia coli O5:K4(L):H4 str. ATCC 23502 Taxon ID: 1268238 | 441652218 | URP | |
| Escherichia coli O10:K5(L):H4 str. ATCC 23506 Taxon ID: 1246623 | 441607795 | URP | |
| Escherichia coli Taxon ID: 562 | 412970373 | URP | |
| Escherichia coli c7122 Taxon ID: 475609 | 412963880 | URP | |
| Escherichia coli str. K-12 substr. MDS42 Taxon ID: 1110693 | 359332826 | URP | |
| Escherichia coli DH1 Taxon ID: 536056 | 315137141 | URP | |
| Escherichia coli ETEC H10407 Taxon ID: 316401 | 309702849 | URP | |
| Escherichia coli 042 Taxon ID: 216592 | 284922467 | URP | |
| Escherichia coli SE15 Taxon ID: 431946 | 281179571 | URP | |
| Escherichia coli BW2952 Taxon ID: 595496 | 259491986 | URP | |
| Escherichia coli O103:H2 str. 12009 Taxon ID: 585395 | 257760294 | URP | |
| Escherichia coli O26:H11 str. 11368 Taxon ID: 573235 | 257755260 | URP | |
| Escherichia coli SE11 Taxon ID: 409438 | 254807180 | URP | |
| Escherichia coli IAI1 Taxon ID: 585034 | 254807178 | URP | |
| Escherichia coli ED1a Taxon ID: 585397 | 254807177 | URP | |
| Escherichia coli IAI39 Taxon ID: 585057 | 254807176 | URP | |
| Escherichia coli 55989 Taxon ID: 585055 | 254807174 | URP | |
| Escherichia coli S88 Taxon ID: 585035 | 254807173 | URP | |
| Escherichia coli O127:H6 str. E2348/69 Taxon ID: 574521 | 254807172 | URP | |
| Escherichia coli BL21(DE3) Taxon ID: 469008 | 242378115 | URP | |
| Escherichia coli LF82 Taxon ID: 591946 | 222034228 | URP | |
| Escherichia coli ED1a Taxon ID: 585397 | 218428201 | URP | |
| Escherichia coli IAI39 Taxon ID: 585057 | 218371013 | URP | |
| Escherichia coli S88 Taxon ID: 585035 | 218366212 | URP | |
| Escherichia coli IAI1 Taxon ID: 585034 | 218361810 | URP | |
| Escherichia coli 55989 Taxon ID: 585055 | 218352876 | URP | |
| Escherichia coli O127:H6 str. E2348/69 Taxon ID: 574521 | 215265939 | URP | |
| Escherichia coli SE11 Taxon ID: 409438 | 209913253 | URP | |
| Shigella boydii CDC 3083-94 Taxon ID: 344609 | 205829888 | URP | |
| Escherichia coli SMS-3-5 Taxon ID: 439855 | 205829754 | URP | |
| Escherichia coli ATCC 8739 Taxon ID: 481805 | 205829753 | URP | |
| Escherichia coli APEC O1 Taxon ID: 405955 | 205829752 | URP | |
| Escherichia coli str. K-12 substr. DH10B Taxon ID: 316385 | 205829750 | URP | |
| Escherichia coli E24377A Taxon ID: 331111 | 205829749 | URP | |
| Shigella sonnei Ss046 Taxon ID: 300269 | 123773477 | URP | |
| Shigella boydii Sb227 Taxon ID: 300268 | 123755559 | URP | |
| Shigella dysenteriae Sd197 Taxon ID: 300267 | 123728531 | URP | |
| Escherichia coli 536 Taxon ID: 362663 | 123147826 | URP | |
| Escherichia coli UTI89 Taxon ID: 364106 | 123084401 | URP | |
| Escherichia coli str. K-12 substr. W3110 Taxon ID: 316407 | 1799916 | ||
| Escherichia coli K-12 Taxon ID: 83333 | 549552 | PRP URP | |
| obsolete GIs = 237705027, 194437563, 194427328, 193064109, 191172618, 191167679, 188491812, 300940232, 300930157, 300927120, 300904309, 300820813, 300817713, 293446870, 293410931, 421462443, 386281584, 332278330, 331684165, 331678508, 331673972, 331669263, 331664080, 331658662, 331653945, 331648214, 331643137, 312965433, 309794436, 309784732, 307312488, 306814413, 301648272, 301330392, 301302874, 301024749, 301022377, 300998073, 300958853, 300951778, 403496657, 375322871, 414577238, 415840204, 415828718, 415815169, 415803712, 415784568, 415778573, 417299867, 417290622, 417286506, 417280820, 417278205, 417272691, 417269007, 417261683, 417251892, 417237952, 417231146, 417221985, 417185071, 417175320, 417163553, 417155465, 417146392, 417140771, 417135332, 417115193, 417085917, 416898710, 416344049, 416335671, 416299209, 416283497, 416268576, 415874042, 415862465, 415844083, 429785680, 429776953, 429772008, 429720107, 427810194, 427805698, 425423361, 425380693, 425306247, 425301372, 425289655, 425284212, 425278918, 425273667, 425120759, 425115994, 424764628, 424754455, 423706637, 423704104, 423061254, 423054279, 423045740, 423044012, 423038892, 423031066, 423025246, 423020080, 423010852, 423004352, 423000680, 422995535, 422988643, 422972902, 422837412, 422835597, 422830004, 422819331, 422799830, 422792447, 422787134, 422780582, 422777776, 422771579, 422767166, 422761951, 422755899, 422752155, 422380266, 422377482, 422367766, 422358132, 422351899, 422333549, 421774685, 421683601, 420392263, 420386570, 420381539, 420363922, 420359717, 420353933, 420348433, 420337236, 420326674, 420134514, 420124623, 420119922, 420113629, 420111510, 420105077, 419950859, 419947494, 419939154, 419931193, 419920343, 419914745, 419906599, 419899550, 419885683, 419878645, 419872747, 419865991, 419810226, 419803528, 419701361, 419413671, 419408081, 419402962, 419397620, 419392635, 419387130, 419381786, 419376450, 419371001, 419366035, 419361141, 419356047, 419350624, 419346164, 419340956, 419335579, 419329946, 419323957, 419317808, 419312372, 419307362, 419301234, 419295839, 419290445, 419285217, 419279050, 419273750, 419268253, 419262309, 419255999, 419250173, 419244362, 419238885, 419233595, 419227872, 419216607, 419210746, 419192563, 419187264, 419181817, 419176148, 419171186, 419165320, 419160204, 419154916, 419149288, 419143467, 419040669, 419035279, 419029983, 419024946, 419019446, 419014428, 419008754, 419003071, 418997594, 418957110, 418941814, 418303966, 418267013, 418041587, 417975814, 417943679, 417863144, 417833812, 417806068, 417756774, 417683190, 417667919, 417663074, 417640270, 417635489, 417629816, 417624472, 417619101, 417613983, 417603171, 417597826, 417582020, 417309002, 432481867, 432471844, 432466706, 432450649, 432447089, 432441983, 432422869, 432417998, 432407598, 432398473, 432393029, 432388027, 432382212, 432377727, 432370740, 432363616, 432358858, 429955118, 429951769, 429944210, 429941530, 429935850, 429929311, 429925374, 429919554, 429914524, 429908653, 429904515, 429823140, 429817928, 429812407, 429807507, 429798994, 429792418, 429791570, 474596421, 474563456, 474505803, 474491556, 459089860, 459078906, 459060369, 459015596, 459010431, 458988513, 458980577, 458973599, 458956522, 458946379, 458916449, 458888448, 458809599, 458684485, 458628587, 458574492, 458467944, 458457035, 458390692, 458375127, 458319617, 458198302, 458175974, 458168670, 458079962, 458061245, 458054862, 458021669, 457977556, 457906447, 450246570, 450219669, 450191399, 442599048, 442592524, 433322937, 433204186, 433199174, 433194574, 433189257, 433184221, 433174419, 433169485, 433164411, 433154598, 433145120, 433135727, 433131059, 433121076, 433111758, 433106782, 433102057, 433097338, 433092891, 433083431, 433078697, 433073746, 433063957, 433048933, 433044069, 433039522, 433034343, 433029455, 433024371, 433014799, 433008576, 433005980, 432991624, 432986233, 432981896, 432972676, 432968596, 432962782, 432956071, 432948364, 432938859, 432935414, 432928093, 432920496, 432905846, 432899594, 432895491, 432876262, 432869853, 432852175, 432840309, 432832574, 432828007, 432821869, 432816244, 432810186, 432806697, 432802712, 432799667, 432793708, 432788423, 432779421, 432771469, 432765875, 432760341, 432755351, 432750966, 432746516, 432742258, 432738008, 432733259, 432728574, 432723993, 432719665, 432714257, 432711537, 432705295, 432699951, 432695336, 432692465, 432686343, 432681127, 432675641, 432671622, 432661728, 432656680, 432647045, 432637759, 432628141, 432622748, 432617659, 432612382, 432603148, 432598523, 432588799, 432584732, 432576780, 432574617, 432564807, 432559732, 432554564, 432549593, 432544102, 432527279, 432514823, 432501009, 432490285, 432486299, 514489527, 557572207, 557567898, 548763129, 548760616, 560158373, 618838211, 608098173, 662006055, 618834118, 556552457, 544391081, 410481491, 407470413, 387830414, 387608167, 386710407, 386705782, 386700537, 386620124, 386609919, 386603443, 386600495, 386594714, 383179527, 378712022, 254289252, 253772589, 251785842, 238901682, 209919994, 170683806, 157157838, 117624746, 110642682, 91211843, 82544966, 74313043, 471334189, 443618572, 388478553, 387622217, 387613113, 386625254, 386615220, 260856611, 260845147, 254162491, 222157223, 218696144, 218690637, 218559443, 218555042, 215487867, 187731368, 170082127, 170019200, 480323726, 544576993, 338769630 | |||
| Show All | |||
Uniprot
| Protein Name | Accession | EC Number
 |
Identifier |
|---|---|---|---|
| Dual-specificity RNA methyltransferase RlmN {ECO:0000255|HAMAP-Rule:MF_01849} | A7ZPW0 | RLMN_ECO24 (Swiss-Prot) | |
| Dual-specificity RNA methyltransferase RlmN {ECO:0000255|HAMAP-Rule:MF_01849} | B7UGW3 | RLMN_ECO27 (Swiss-Prot) | |
| Dual-specificity RNA methyltransferase RlmN {ECO:0000255|HAMAP-Rule:MF_01849} | B7MI02 | RLMN_ECO45 (Swiss-Prot) | |
| Dual-specificity RNA methyltransferase RlmN {ECO:0000255|HAMAP-Rule:MF_01849} | B7LDA9 | RLMN_ECO55 (Swiss-Prot) | |
| Dual-specificity RNA methyltransferase RlmN {ECO:0000255|HAMAP-Rule:MF_01849} | B7NRG7 | RLMN_ECO7I (Swiss-Prot) | |
| Dual-specificity RNA methyltransferase RlmN {ECO:0000255|HAMAP-Rule:MF_01849} | B7N304 | RLMN_ECO81 (Swiss-Prot) | |
| Dual-specificity RNA methyltransferase RlmN {ECO:0000255|HAMAP-Rule:MF_01849} | B7M7M1 | RLMN_ECO8A (Swiss-Prot) | |
| Dual-specificity RNA methyltransferase RlmN {ECO:0000255|HAMAP-Rule:MF_01849} | C4ZX92 | RLMN_ECOBW (Swiss-Prot) | |
| Dual-specificity RNA methyltransferase RlmN {ECO:0000255|HAMAP-Rule:MF_01849} | B1XAZ2 | RLMN_ECODH (Swiss-Prot) | |
| Dual-specificity RNA methyltransferase RlmN {ECO:0000255|HAMAP-Rule:MF_01849} | A1AE55 | RLMN_ECOK1 (Swiss-Prot) | |
| Dual-specificity RNA methyltransferase RlmN {ECO:0000255|HAMAP-Rule:MF_01849} | Q0TEW8 | RLMN_ECOL5 (Swiss-Prot) | |
| Dual-specificity RNA methyltransferase RlmN {ECO:0000255|HAMAP-Rule:MF_01849} | B1IWE4 | RLMN_ECOLC (Swiss-Prot) | |
| Dual-specificity RNA methyltransferase RlmN | P36979 | RLMN_ECOLI (Swiss-Prot) | |
| Dual-specificity RNA methyltransferase RlmN {ECO:0000255|HAMAP-Rule:MF_01849} | B6I589 | RLMN_ECOSE (Swiss-Prot) | |
| Dual-specificity RNA methyltransferase RlmN {ECO:0000255|HAMAP-Rule:MF_01849} | B1LNH2 | RLMN_ECOSM (Swiss-Prot) | |
| Dual-specificity RNA methyltransferase RlmN {ECO:0000255|HAMAP-Rule:MF_01849} | Q1R8L6 | RLMN_ECOUT (Swiss-Prot) | |
| Dual-specificity RNA methyltransferase RlmN {ECO:0000255|HAMAP-Rule:MF_01849} | B2TXU2 | RLMN_SHIB3 (Swiss-Prot) | |
| Dual-specificity RNA methyltransferase RlmN {ECO:0000255|HAMAP-Rule:MF_01849} | Q31XX3 | RLMN_SHIBS (Swiss-Prot) | |
| Dual-specificity RNA methyltransferase RlmN {ECO:0000255|HAMAP-Rule:MF_01849} | Q32D45 | RLMN_SHIDS (Swiss-Prot) | |
| Dual-specificity RNA methyltransferase RlmN {ECO:0000255|HAMAP-Rule:MF_01849} | Q3YZ35 | RLMN_SHISS (Swiss-Prot) | |
| Show All | |||
Length of Enzyme (full-length): 384 | Length of Functional Domain: 365
MSEQLVTPENVTTKDGKINLLDLNRQQMREFFKDLGEKPFRADQVMKWMYHYCCDNFDEM
TDINKVLRGKLKEVAEIRAPEVVEEQRSSDGTIKWAIAVGDQRVETVYIPEDDRATLCVS
SQVGCALECKFCSTAQQGFNRNLRVSEIIGQVWRAAKIVGAAKVTGQRPITNVVMMGMGE
PLLNLNNVVPAMEIMLDDFGFGLSKRRVTLSTSGVVPALDKLGDMIDVALAISLHAPNDE
IRDEIVPINKKYNIETFLAAVRRYLEKSNANQGRVTIEYVMLDHVNDGTEHAHQLAELLK
DTPCKINLIPWNPFPGAPYGRSSNSRIDRFSKVLMSYGFTTIVRKTRGDDIDAACGQLAG
DVIDRTKRTLRKRMQGEAIDIKAV
Conserved catalytic residues (as determined by automated alignment to family, subgroup, or superfamily HMMs) are shown with teal highlighting . Conserved catalytic residues which do not matched the Conserved Alignment Residue are shown with maroon highlighting . Information regarding their function can be found in the Conserved Residues section below.